Isotope psychoactive substance labeled compound and preparation method and application thereof
A technology of active substances and compounds, which is applied in the field of preparation and application of reference substances of psychoactive substances, and can solve the problems of low detection accuracy and sensitivity of psychoactive substances
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
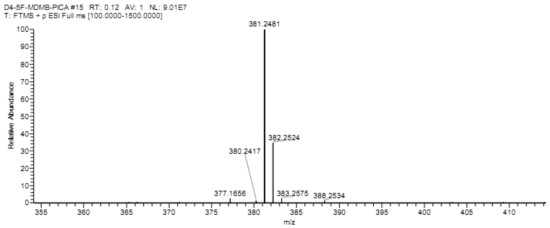
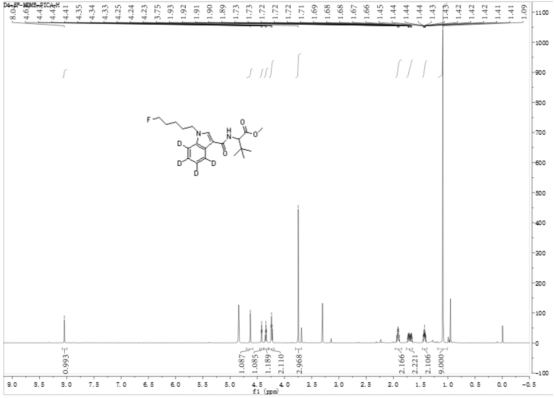
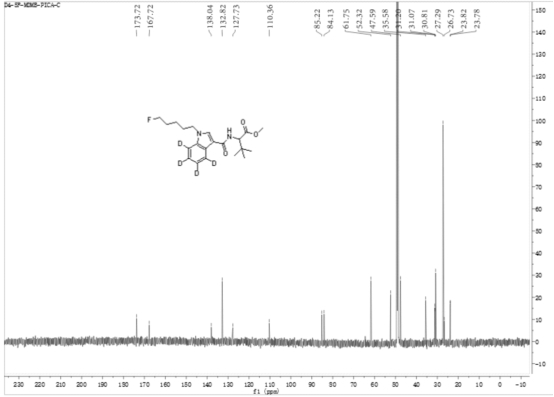
[0059] 2-(1-(5-fluoropentyl)-indole-3-amide)--methyl 4,5,6,7-d4-3,3-dimethylbutanoate (D4-5F-MDMB- PICA) preparation method.
[0060] At -20°C, N-(formyloxy) tert-leucine methyl ester (567 mg, 3 mmol) was dissolved in 50 mL of anhydrous anhydrous carbon tetrachloride, and aluminum trichloride (400 mg, 3 mmol), then N-5-fluoropentylindole (200 mg, 1 mmol) in anhydrous carbon tetrachloride solution (25 mL) was added dropwise, stirred and slowly warmed to room temperature, and kept at room temperature for 12 hours Heat to 40 °C and heat for 2 hours. After cooling, add ammonium chloride solution to quench the reaction, remove the solvent by rotary evaporation, add 150 mL ethyl acetate to dissolve, remove insoluble matter by diatomaceous earth filtration, wash diatomaceous earth with 30 mL diethyl ether-ethyl acetate 1:1 solution, The organic phases were combined, washed with 5% hydrochloric acid aqueous solution, and the aqueous phase was extracted with ethyl acetate, and the co...
Embodiment 2
[0062] With 2-(1-(5-fluoropentyl)-indole-3-amide)-4,5,6,7-d4-3,3-dimethylbutanoic acid methyl ester (D4-5F-MDMB- PICA) was used as the internal standard to detect the content of 2-(1-(5-fluoropentyl)-indole-3-amide)-3,3-dimethylbutyric acid methyl ester (5F-MDMB-PICA) in hair.
[0063] (1) Liquid chromatography-mass spectrometry detection conditions:
[0064] a) Instrument model: Agilent 1290-6470QQQ;
[0065] b) Column: Poroshell120 PFP 3.0 x100mm 1.9um;
[0066] c) Column temperature: 30°C;
[0067] d) Mobile phase: A: acetonitrile (0.01% formic acid), B: water (0.01% formic acid, 5% ammonium formate), gradient: 1min 10%A, 2min 50%A, 4min 90%A, 6min 95%A;
[0068] e) Flow rate: 0.5 mL / min;
[0069] f) Injection volume: 5 μL;
[0070] g) Ion source: electrospray ion source, positive mode (ESI+);
[0071] h) The spray voltage is 3500 V;
[0072] i) Ion source temperature: 340°C;
[0073] j) Collision gas: nitrogen;
[0074] The ion pairs and corresponding conditions a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com