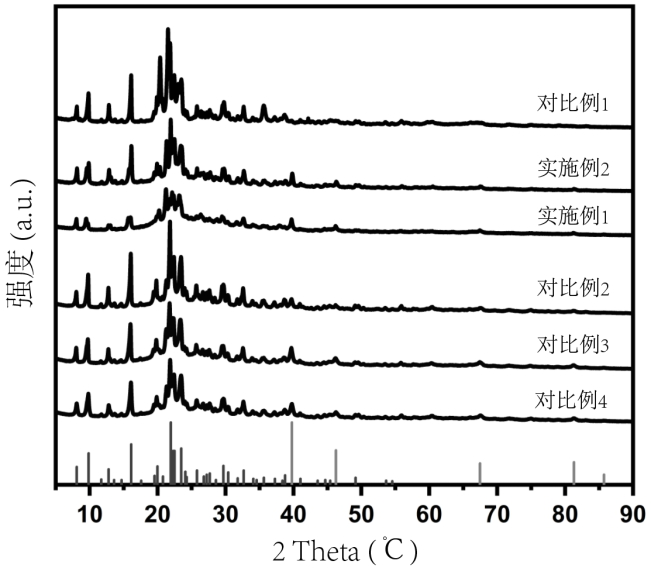
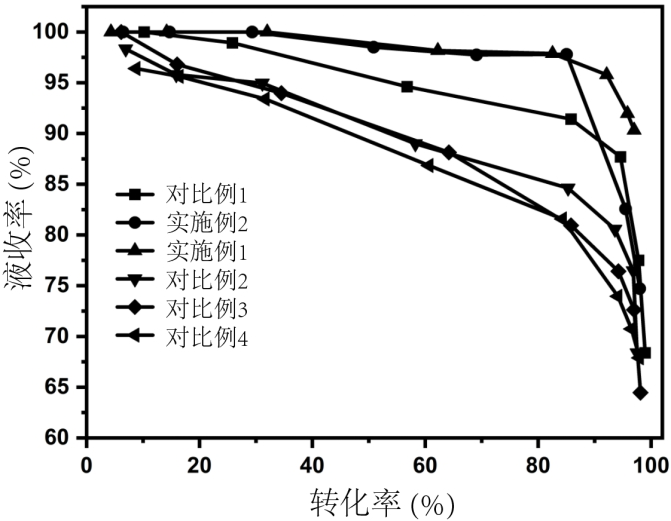
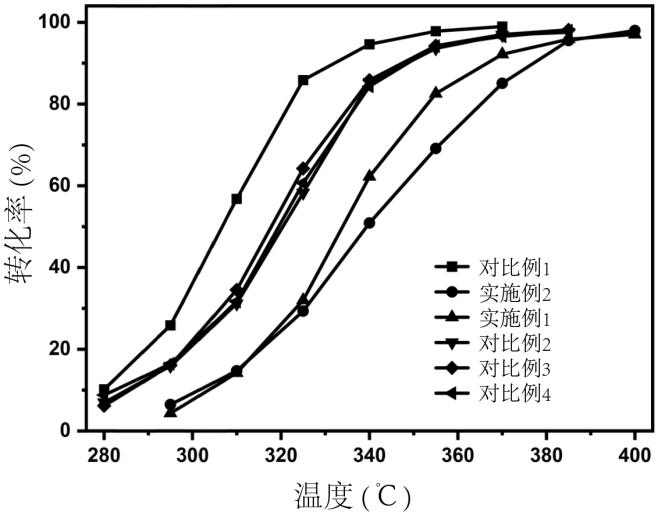
Preparation method and application of biomass charcoal assisted synthesis bifunctional catalyst
A dual-function catalyst, biomass carbon technology, applied in catalyst activation/preparation, physical/chemical process catalysts, molecular sieve catalysts, etc., can solve the problems of low catalyst selectivity, long-term storage of precious metal nanocatalysts, and complex preparation processes, etc. Achieve the effect of promoting sustainability and cost reduction, good isomer selectivity and stability, and simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] First, use 1.0 mL of H with a concentration of 0.133 mol / L 2 PtCl 6 The solution was impregnated with 0.283 g of commercially available biomass capacitor carbon powder, maintained at room temperature for 24 h, dried, and calcined to obtain the metal@biomass carbon precursor (M@RS). M@RS was mixed with 0.25 g silica, 2.912 g pseudoboehmite, 6.28 g DPA·H 3 PO 4 Mixed and ground for 5 min, placed in a hydrothermal kettle for crystallization at 185 °C for 24 h, the reacted product was washed by centrifugation, dried at 80 °C and calcined at 600 °C for 5 h in an air atmosphere to obtain the final catalyst product. The samples prepared above were tested by plasma emission spectrometry (ICP), and the metal loading was determined to be 0.5 wt%. The obtained catalyst was named M@RS / S11.
Embodiment 2
[0036] First, use 1.0 mL of H with a concentration of 0.133 mol / L 2 PtCl 6The solution was impregnated with 0.283 g of commercially available coconut shell charcoal powder, maintained at room temperature for 24 h, dried, and calcined to obtain the metal@biomass charcoal precursor (M@CS). Mix M@CS with 0.25 g silica, 2.912 g pseudoboehmite, 6.28 g DPA·H 3 PO 4 Mixed and ground for 5 min, placed in a hydrothermal kettle for crystallization at 185 °C for 24 h, the reacted product was washed by centrifugation, dried at 80 °C and calcined at 600 °C for 5 h in an air atmosphere to obtain the final catalyst product. The samples prepared above were tested by plasma emission spectrometry (ICP), and the metal loading was determined to be 0.5 wt%. The obtained catalyst was named M@CS / S11.
Embodiment 3
[0038] First, use 1.0 mL of H with a concentration of 0.133 mol / L 2 PtCl 6 The solution was impregnated with 0.283 g of commercially available walnut shell carbon powder, maintained at room temperature for 24 h, dried, and calcined to obtain the metal@biomass carbon precursor (M@WS). M@WS was mixed with 0.25 g silica, 2.912 g pseudoboehmite, 6.28 g DPA·H 3 PO 4 Mixed and ground for 5 min, placed in a hydrothermal kettle for crystallization at 185 °C for 24 h, the reacted product was washed by centrifugation, dried at 80 °C and calcined at 600 °C for 5 h in an air atmosphere to obtain the final catalyst product. The samples prepared above were tested by plasma emission spectrometry (ICP), and the loading amount of metal was measured to be 0.5wt%. The obtained catalyst was named M@WS / S11.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com