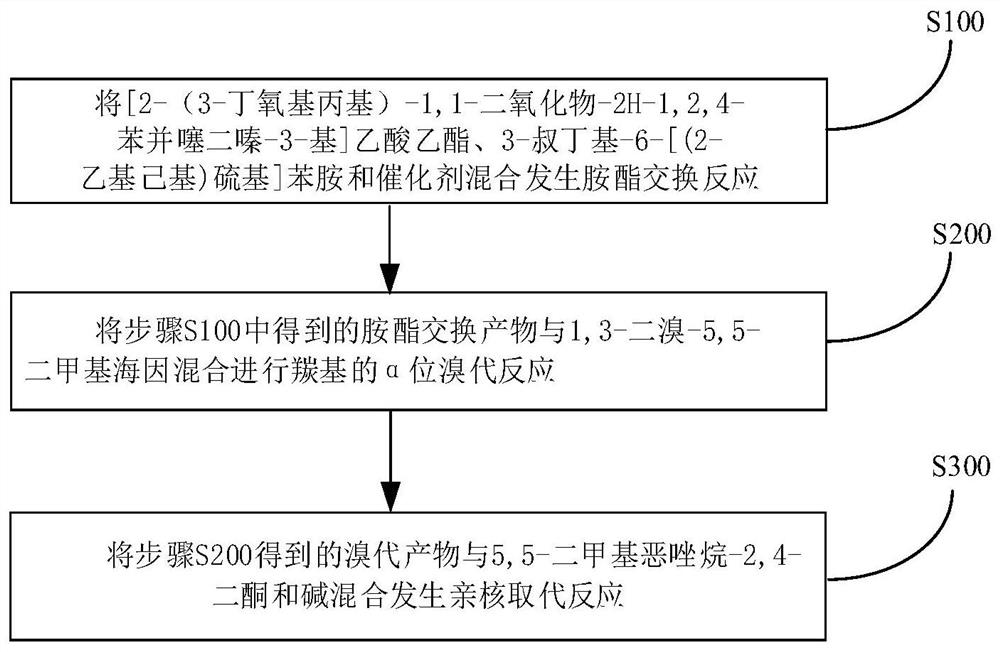
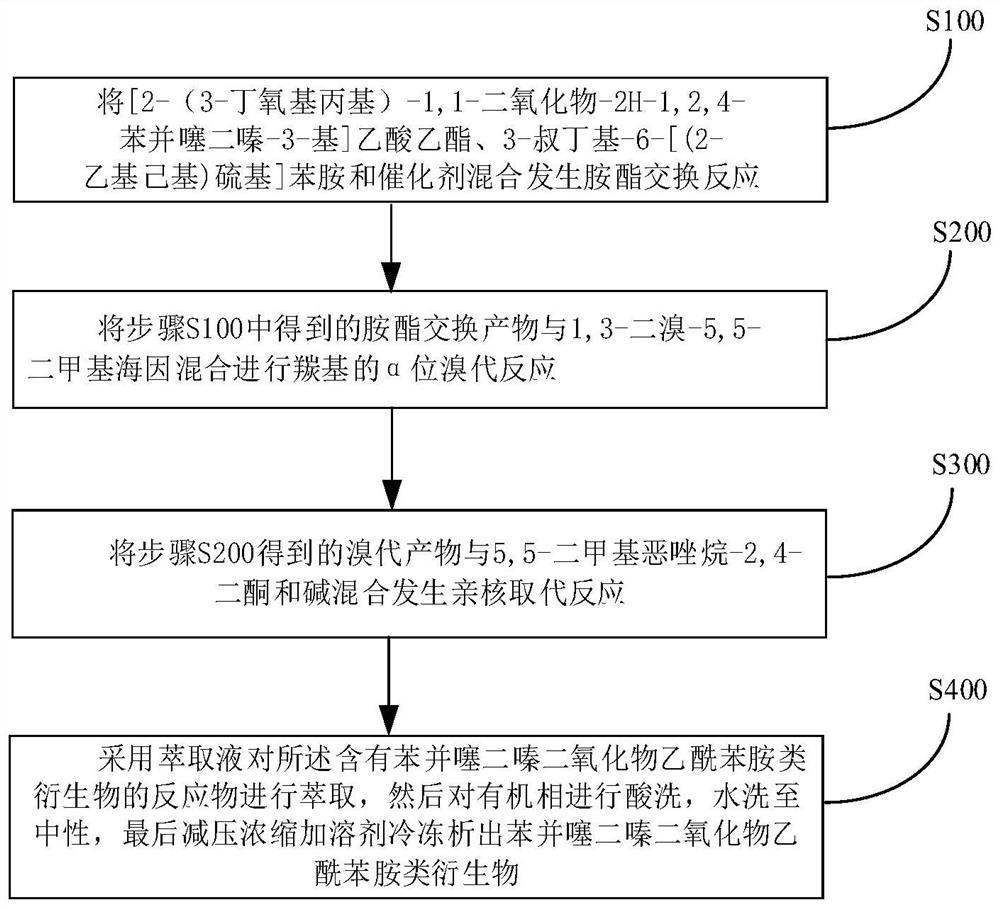
Method for synthesizing benzothiadiazine dioxide acetanilide derivative by one-pot method
A technology of benzothiadiazine and dioxide, which is applied in the field of one-pot synthesis of benzothiadiazine dioxide acetanilide derivatives, can solve the problem of increasing the complexity of process operations, waste water and liquid discharge, and increasing labor costs. Cost and environmental protection costs, synthetic methods need to be improved, etc., to achieve the effect of reducing use and manual operation costs, optimizing the sequence of addition, and reducing manual operations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
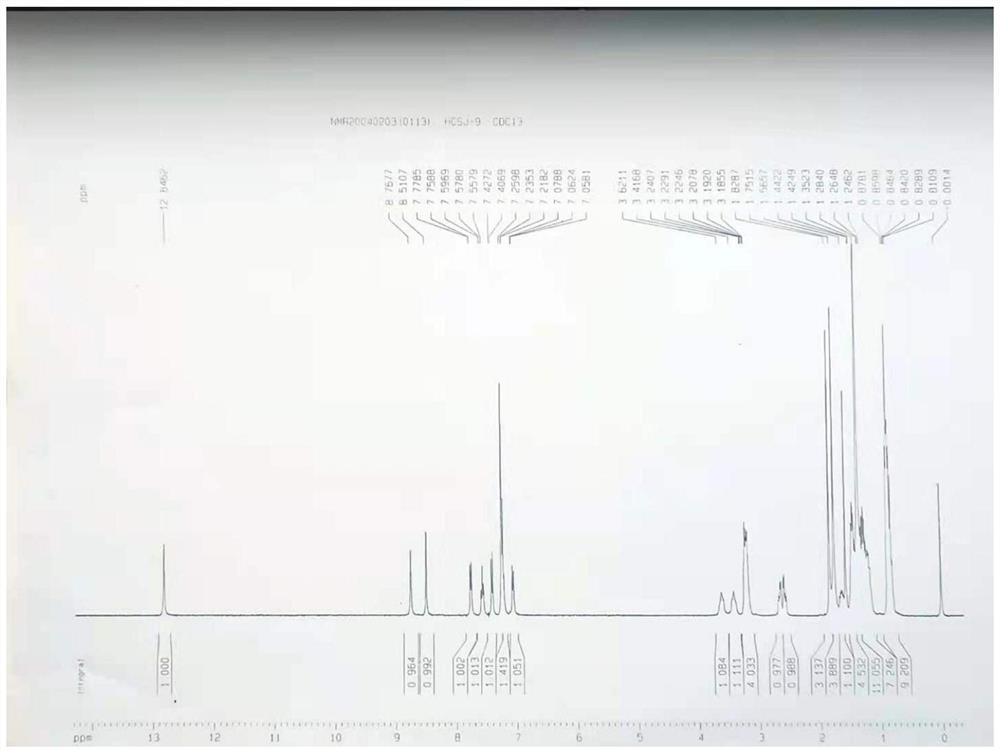
[0051] To a 500mL three-necked flask equipped with a water separator, add 14.67g of 3-tert-butyl-6-[(2-ethylhexyl)thio]aniline, [2-(3-butoxypropyl)-1,1 -dioxide-2H-1,2,4-benzothiadiazin-3-yl]ethyl acetate 19.10g, aluminum isopropoxide 4.08g, anhydrous aluminum trichloride 0.67g and xylene 30mL, 150 After reacting at ℃ for 2 hours, the temperature was raised to 160℃. After reacting for 5 hours, 100mL of toluene was added. At 5℃, 7.15g of 1,3-dibromo-5,5-dimethylhydantoin was added in batches. After reacting for 1.5h, 5, 5-Dimethyloxazolidine-2,4-dione 16.45g, K 2 CO 3 6.9g, reacted at 60°C for 2h, added toluene and water to extract and separate the liquid after the reaction, washed the organic phase once with dilute acid, washed with water until neutral, concentrated under reduced pressure and added methanol to freeze to precipitate 28.61g of solid. The NMR characterization of the final compound is shown in image 3 shown.
Embodiment 2
[0053] To a 500mL three-necked flask equipped with a water separator, add 16.87g of 3-tert-butyl-6-[(2-ethylhexyl)thio]aniline, [2-(3-butoxypropyl)-1,1 -dioxide-2H-1,2,4-benzothiadiazin-3-yl]ethyl acetate 19.10g, aluminum isopropoxide 4.08g, titanium chloride 0.95g and xylene 30mL, 155°C reaction 2 After 1 hour, the temperature was raised to 165°C. After 5 hours of reaction, 100 mL of toluene was added. At 15°C, 8.58 g of 1,3-dibromo-5,5-dimethylhydantoin was added in batches. After 1.5 hours of reaction, 5,5-Dimethylhydantoin was added. Methyloxazolidine-2,4-dione 8.38g, Na 2 CO 3 8.96g, react at 60°C for 2h, add toluene and water to extract and separate the liquid after the reaction, wash the organic phase once with dilute acid, wash with water until neutral, concentrate under reduced pressure and add ethanol to freeze to precipitate 29.67g of solid. The NMR characterization of the final compound is shown in image 3 shown.
Embodiment 3
[0055] To a 500mL three-necked flask equipped with a water separator, add 16.14g of 3-tert-butyl-6-[(2-ethylhexyl)thio]aniline, [2-(3-butoxypropyl)-1,1 -dioxide-2H-1,2,4-benzothiadiazin-3-yl]ethyl acetate 19.10g, aluminum isopropoxide 4.08g and xylene 30mL, react at 150°C for 2 hours and then heat up to 165°C After 5 hours of reaction, 100 mL of toluene was added, 7.86 g of 1,3-dibromo-5,5-dimethylhydantoin was added in batches at 25°C, and 5,5-dimethyloxazolidine- 2,4-diketone 7.74g, NaHCO 3 6.05g, reacted at 60°C for 2h, added toluene and water after the reaction, extracted and separated the liquid, washed the organic phase once with dilute acid, washed with water until neutral, concentrated under reduced pressure and added acetonitrile to freeze to precipitate 28.92g of solid. The NMR characterization of the final compound is shown in image 3 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com