Novel HPK1 inhibitor, and preparation method and application thereof
A selected and unsubstituted technology applied in the field of small molecule drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
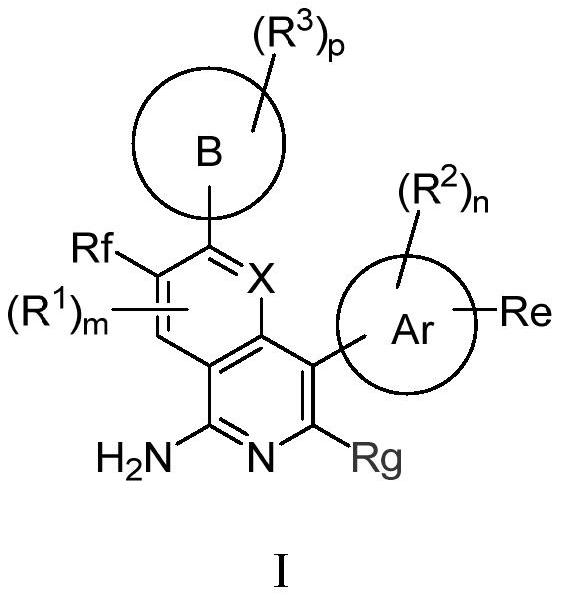
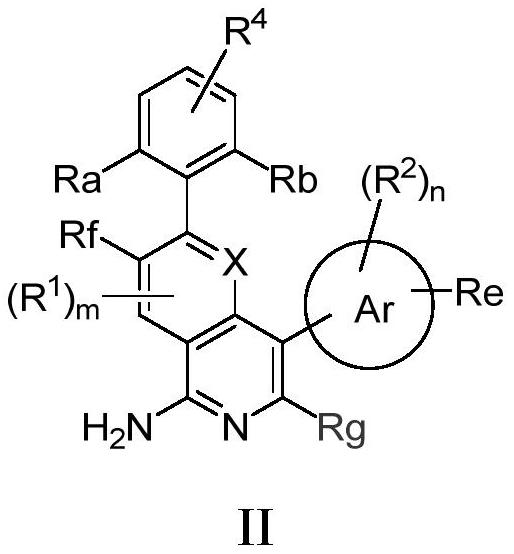
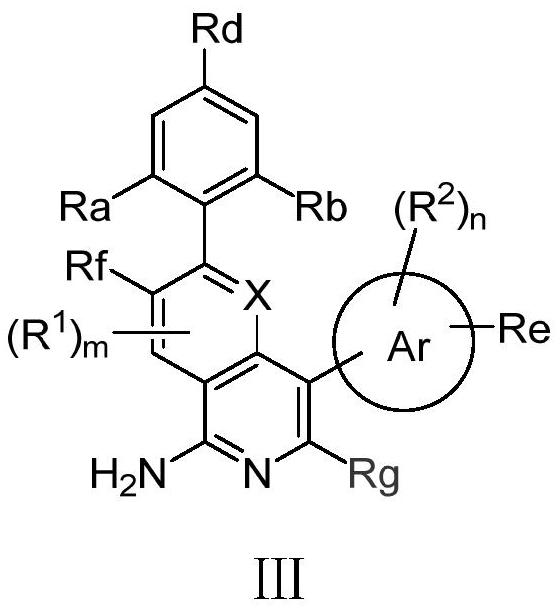
[0082] The preparation of formula I compound
[0083] Compounds of formula I of the present invention can be prepared by the following methods:
[0084]
[0085] In an inert solvent, react with a brominated reagent and boronic acid to obtain a compound of formula I;
[0086] or
[0087]
[0088] In an inert solvent, a brominated reagent is used to react with a boric acid ester to obtain a compound of formula I.
[0089] Pharmaceutical compositions and methods of administration
[0090] Since the compound of the present invention has excellent HPK1 kinase inhibitory activity, the compound of the present invention and its various crystal forms, pharmaceutically acceptable inorganic or organic salts, hydrates or solvates, and compounds containing the compound of the present invention as the main active ingredient The pharmaceutical composition of can be used to prevent and / or treat diseases related to HPK1 kinase activity or expression level (for example, cancer).
[00...
Embodiment 1
[0109] Example 1: 2-(2-fluoro-6-methylphenyl)-8-(6-(4-methylpiperazin-1-yl)pyridin-3-yl)-1,6-diazepine Xanaphthyl-5-amine
[0110]
[0111] 5-Chloro-1,6-naphthyridine-1-oxide
[0112]
[0113] 5-Chloro-1,6-naphthyridine (500 mg, 3.04 mmol) and chloroform (10 mL) were added to a 50 mL eggplant-shaped flask equipped with a magnetic stirrer, and the reaction solution was placed in an ice bath Let cool for 20 minutes. To the solution was added m-chloroperoxybenzoic acid (purity: 85%, 926 mg, 4.56 mmol) in portions. The reaction mixture was then stirred overnight at room temperature. The reaction was checked by LCMS. After the reaction, the reaction solution was concentrated under reduced pressure to obtain a solid residue, which was separated through a flash silica gel column (dichloromethane:methanol=100:1) to obtain a yellow solid 5-chloro-1,6-naphthyridine- 1-oxide (597 mg, purity: 90%, yield: 100%). MS(ESI):m / z=181.0,183.0[M+H] + .
[0114] 5-Chloro-1,6-nap...
Embodiment 2
[0135] Example 2: 1-amino-6-(2-fluoro-6-methylphenyl)-4-(6-(4-methylpiperazin-1-yl)pyridin-3-yl)iso Quinoline-7-carbonitrile
[0136]
[0137] 6-Chloroisoquinoline-7-carbonitrile
[0138]
[0139] Under the protection of argon, the N,N-dimethylformamide (15 mL) into the mixture, tetrakis-triphenylphosphopalladium (457 mg, 0.39 mmol) was added, and the reaction solution was heated to 100° C. and stirred for 2 hours. The reaction was checked by LCMS. After the reaction was completed, the reaction liquid was cooled to room temperature, the reaction liquid was poured into water (50 ml), and then extracted with ethyl acetate (20 ml × 2), the organic layers were combined, followed by water (20 ml × 2), saturated Wash with brine (20 ml), dry over anhydrous sodium sulfate, filter, and concentrate the filtrate under reduced pressure to obtain a crude product. The crude product was separated and purified by flash silica gel column (petroleum ether:ethyl acetate=5:1) to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com