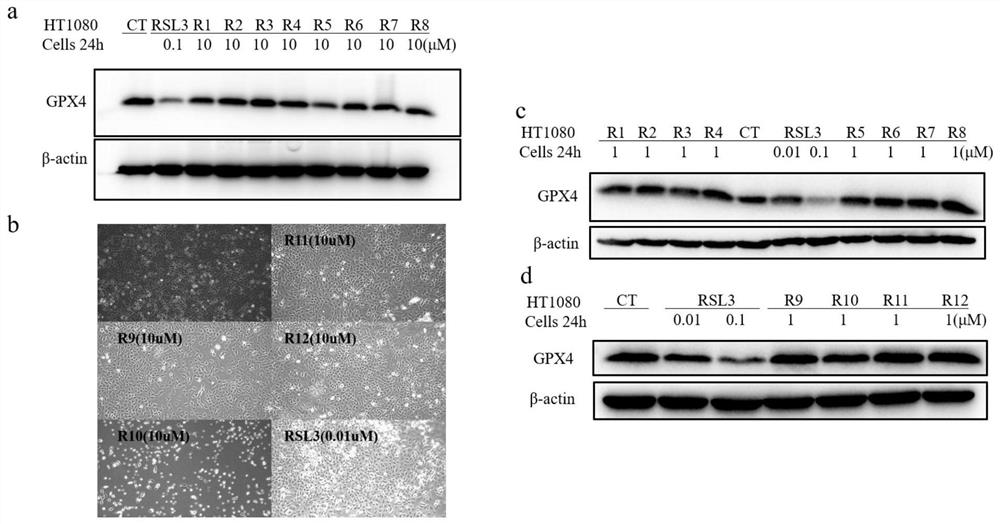
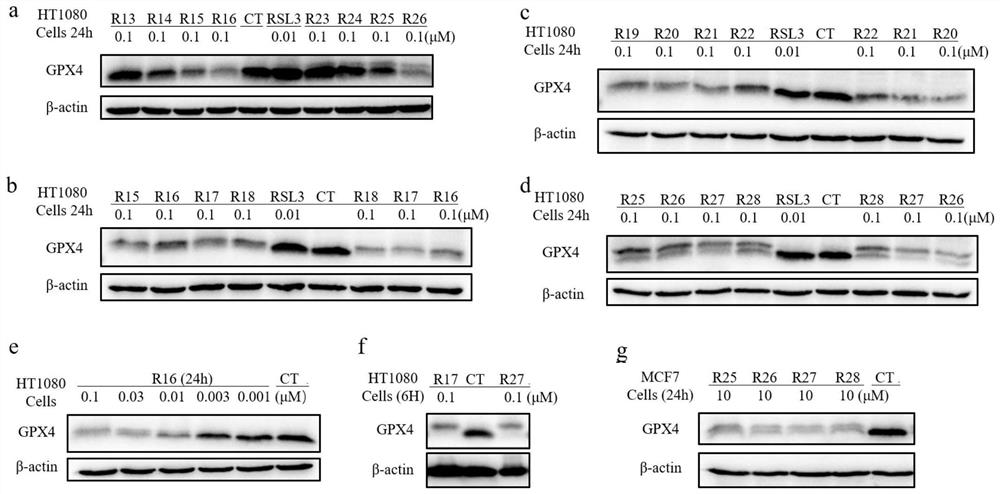
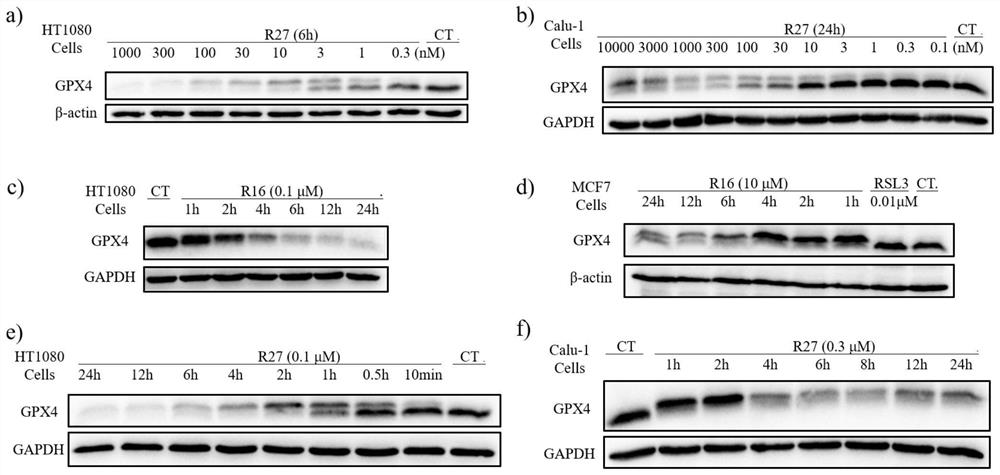
GPX4 protein degradation agent, preparation method and application thereof, and antitumor cell drug
A protein degradation and reaction technology, applied in the field of anti-tumor cell drugs, to achieve good anti-proliferation effect, good GPX4 degradation effect, and good biological activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0128] The present invention provides the preparation method of above-mentioned GPX4 protein degradation agent, comprises the following steps:
[0129] ①When A 1 for hour,
[0130] The preparation method of described GPX4 protein degradation agent comprises the following steps:
[0131] The E3 ligand compound substituted by the azidopolyethylene glycol chain reacts with the first GPX4 ligand to obtain a GPX4 protein degradation agent;
[0132] The azido polyalcohol chain heterocyclic compound has a structure shown in formula A-1, A-2 or A-3:
[0133]
[0134] The first GPX4 ligand has a structure shown in formula B:
[0135]
[0136] In the present invention, the molar ratio of the E3 ligand compound substituted by the azidopolyethylene glycol chain to the first GPX4 ligand is preferably 0.11:0.12; in the present invention, the catalyst for the ring-forming reaction is preferably For sodium ascorbate and copper sulfate, the molar ratio of the sodium ascorbate, copper...
Embodiment 1
[0274] The synthesis of embodiment 1GPX4 protein degradation agent R1
[0275] (1) Synthesis of the first GPX4 ligand
[0276] a. Synthesis of compound shown in formula g
[0277] Dissolve p-formylbenzoic acid (8.00g, 53.29mmol), propiolic acid (4.40g, 79.93mmol) in THF (120mL), then add EDCI (15.32g, 79.93mmol), HOBT (10.80g, 79.93mmol) ), reacted at room temperature for 14h. After the reaction, part of the solvent was rotated off under reduced pressure, extracted three times with water and ethyl acetate, the organic phases were combined, washed with saturated NaCl solution and anhydrous NaCl 2 SO 4 dry. Column separation (P / E=5 / 1, 2 / 1) gave a white solid, and the compound represented by formula g was obtained, which was designated as RSL3-Q1. 1 H NMR (400MHz, DMSO-d 6 )δ10.09(s, 1H, CHO), 9.17(t, J=2.68Hz, 1H, CONH), 7.80-8.06(m, 4H, Ar-H), 4.09(dd, J=5.52, 2.48Hz, 2H, CH 2 ), 3.16(t, J=2.48Hz, 1H, CH); 13 CNMR (400MHz, DMSO-d 6 )δ193.34, 165.61, 139.20, 138.39, 12...
Embodiment 2~4
[0291] The synthesis of embodiment 2~4GPX4 protein degradation agent R2~4
[0292] The difference between Examples 2-4 and Example 1 lies in the difference in the n value of the starting material when preparing the compound having the structure shown in the formula PEG1-7.
[0293] Spectral data of R2: 1 H NMR (400MHz, DMSO-d 6 )δ10.93(s, 1H, NH), 8.91(t, J=7.76Hz, 1H, CONH), 7.74-7.89(m, 3H, Ar-H), 7.46-7.61(m, 3H, Ar-H ), 7.18-7.30(m, 2H, Ar-H), 6.92-7.08(m, 3H, Ar-H), 6.81(d, J=7.88Hz, 1H, Ar-H), 6.02(s, 1H, CH), 5.39 (brs, 1H, CH), 5.18 (dd, J=13.24, 4.92Hz, 1H, CH), 4.72 (d, J=14.00Hz, 1H, CH 2a ), 4.40-4.48 (m, 5H, CH 2 ×2&CH 2b ), 4.11 (q, J=57.08, 16.76Hz, 2H, CH 2 ), 3.73-3.88 (m, 4H, CH 2 ×2), 3.39-3.59(m, 15H, CH 2 ×6&CH 3 ), 2.74-3.04 (m, 2H, CH 2 ), 2.02-2.33 (m, 2H, CH 2 ); 13 CNMR (400MHz, DMSO-d 6 )δ172.13,171.07,169.37,168.20,166.26,159.34,145.53,144.04,136.88,134.27,133.07,132.68,129.31,127.75,126.17,126.12,123.61,123.59,121.81,121.78,119.34,118.48...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com