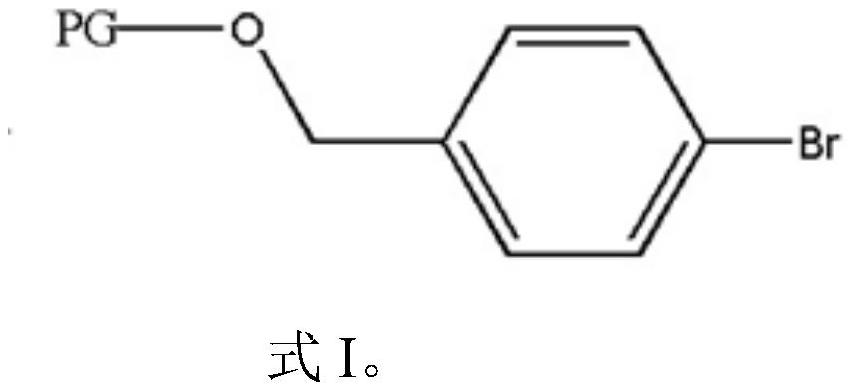
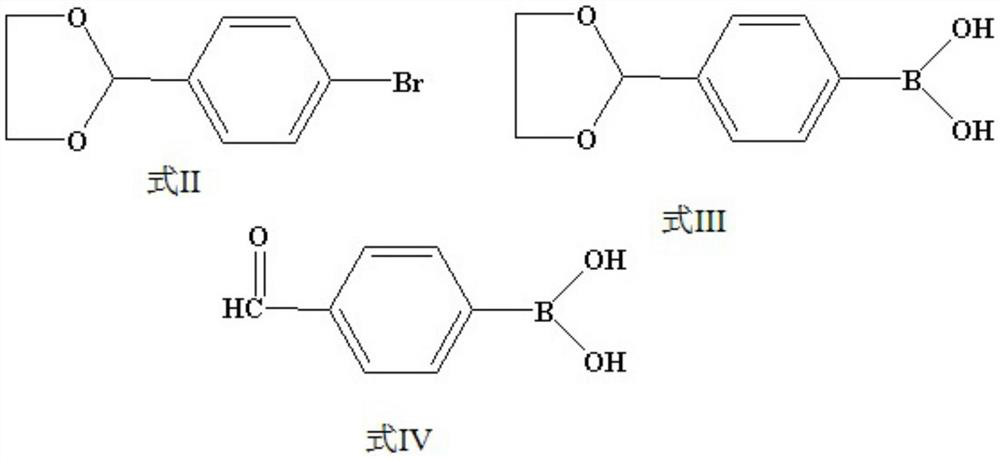
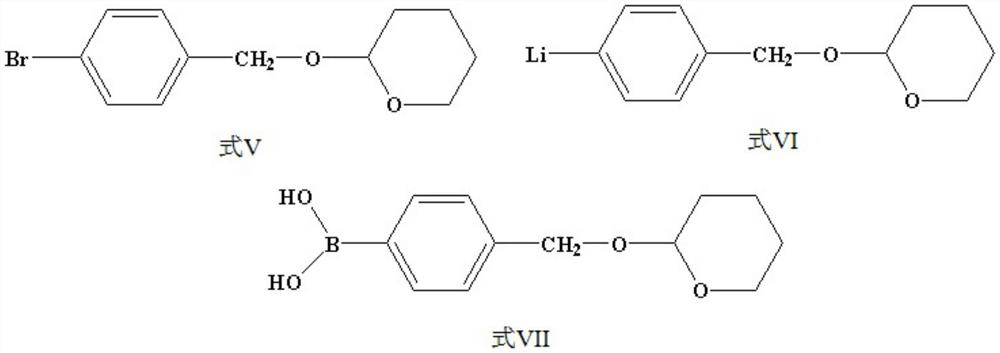
Preparation method of 4-hydroxymethyl phenylboronic acid
A technology of hydroxymethylphenylboronic acid and hydroxyl protection agent, which is applied in the field of preparation of 4-hydroxymethylphenylboronic acid, can solve the problems of high preparation cost, low yield and low purity of 4-hydroxymethylphenylboronic acid, and achieves the The effect of low production cost, environmental friendliness and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The embodiment of the present invention provides a preparation method of 4-hydroxymethylphenylboronic acid, comprising the following steps:
[0057] Step 1. Under the protection of nitrogen, put 93.5g of p-bromobenzyl alcohol and 37.4g of imidazole in a 1000mL three-necked flask, add 270mL of dichloromethane, stir well, and add tert-butyldimethyl chloride dropwise in an ice-water bath at 5°C Silane solution (79.1g of tert-butyldimethylsilyl chloride dissolved in 180mL of dichloromethane), temperature controlled at 0°C to 10°C and added dropwise for 50 minutes, after the dropwise addition was completed, heated to 30°C, reacted for 2 hours, then After treatment, 144.6 g of crude tert-butyldimethylsiloxymethylene bromide represented by formula VIII was obtained, with a yield of 95.1% and a purity of 99.1%;
[0058]
[0059] Step 2, under the protection of nitrogen, dissolve the crude product of tert-butyldimethylsiloxymethylene bromide benzene in 300mL tetrahydrofuran t...
Embodiment 2
[0063] Embodiments of the present invention provide a method for preparing 4-hydroxymethylphenylboronic acid, comprising the following steps:
[0064] Step 1. Under the protection of nitrogen, put 93.5g of p-bromobenzyl alcohol and 75.9g of triethylamine into a 1000mL three-necked flask, add 190mL of tetrahydrofuran, stir well, and add phenyldimethylchlorosilane dropwise in an ice-water bath at 5°C solution (93.9g of phenyldimethylsilyl chloride dissolved in 460mL of tetrahydrofuran), temperature-controlled at 10°C to 15°C and added dropwise for 60 minutes, after the dropwise addition was completed, heated to 40°C, reacted for 4 hours, and post-processed to obtain formula X The crude product phenyldimethylsiloxymethylene bromide benzene 154.3g, yield 95.3%, purity 99.2%;
[0065]
[0066] Step 2, under the protection of nitrogen, dissolve the crude product of phenyldimethylsiloxymethylene bromide benzene in 770mL ether to obtain the ether solution of phenyldimethylsiloxymet...
Embodiment 3
[0070] Embodiments of the present invention provide a method for preparing 4-hydroxymethylphenylboronic acid, comprising the following steps:
[0071] Step 1. Under nitrogen protection, put 93.5g of p-bromobenzyl alcohol and 95.4g of sodium carbonate in a 1000mL three-necked flask, add 370mL of ether, stir well, and add dropwise triethylchlorosilane solution (98.0 g triethylchlorosilane dissolved in 340mL tetrahydrofuran), temperature controlled at 15°C to 25°C and added dropwise for 50min, after the dropwise addition was completed, heated to 30°C, reacted for 2h, post-processed to obtain the crude product III shown in formula XII Ethylsiloxymethylene bromide benzene 144.7g, yield 95.3%, purity 99.2%;
[0072]
[0073] Step 2, under the protection of nitrogen, the above crude triethylsiloxymethylene bromide benzene was dissolved in 430mL dioxane to obtain a dioxane solution of triethylsiloxymethylene bromide benzene, which was then Add dropwise to a 1000mL three-necked fla...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com