A kind of polypeptide and its application in the preparation of anti-platelet aggregation medicine
An anti-platelet aggregation and platelet aggregation technology, which is applied in the field of medicine and biology, can solve the problems of increasing the risk of cerebral hemorrhage in patients and not seeing the activity of polypeptide A11, and achieve the effect of inhibiting platelet aggregation, inhibiting platelet aggregation, and seeing no toxic and side effects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
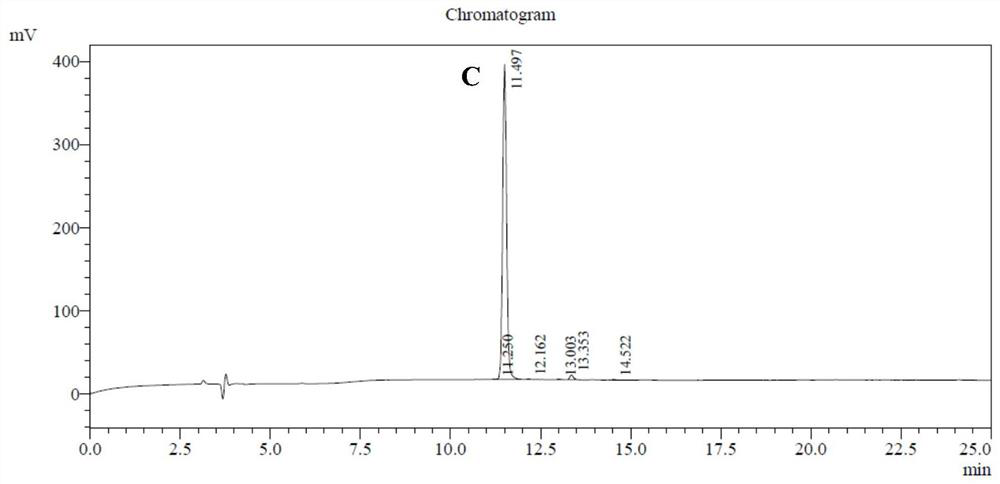
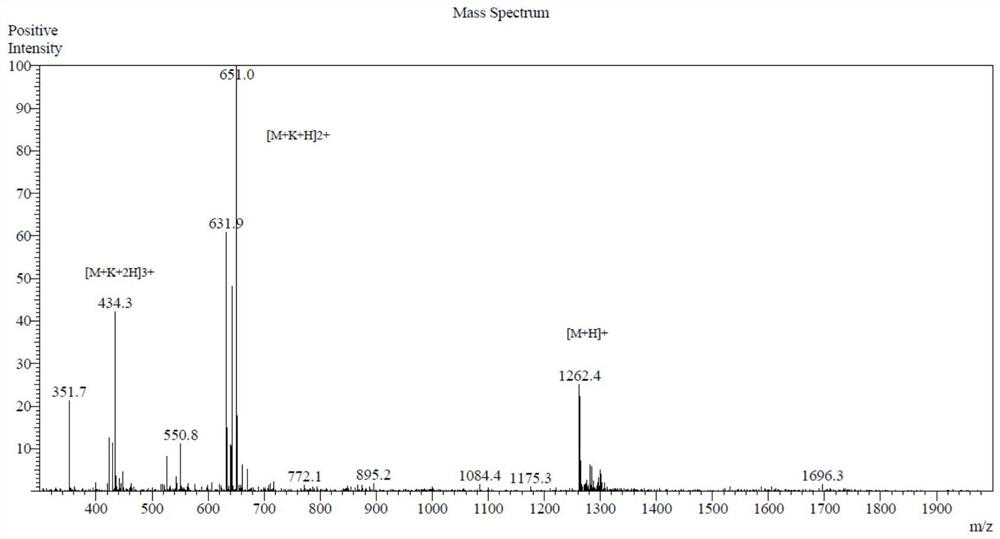
[0030] According to the method reported in the literature (La Face DM, Couture C, Anderson K, et al. Differential T cell signaling induced by antagonist peptide-MHC complexes and the associated phenotypic responses. J Immunol. 1997 Mar 1;158(5):2057–2064 .), the peptide A11 was synthesized using the method of solid-phase peptide synthesis. The synthesized peptides were purified by high performance liquid chromatography (HPLC), and the concentration was ≥95% (attached). figure 1 ). Subsequently, the amino acid sequence and molecular weight were determined by mass spectrometry (MS), and it was confirmed that the synthesized polypeptide was A11 (attached). figure 2 ).
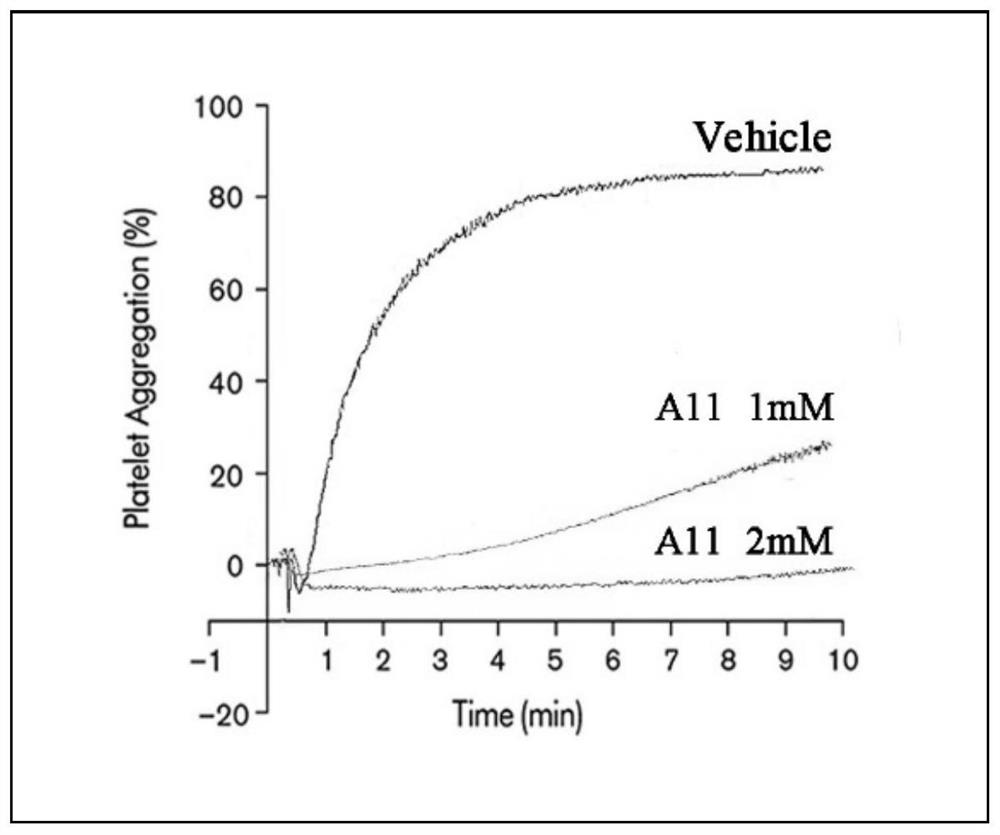
[0031] A11 inhibits thrombin-induced human platelet aggregation in vitro.
[0032] (1) Healthy volunteers from platelets from the experimenter signed the informed consent for blood donation and were given certain nutritional subsidies. Venous whole blood was collected, apheresis platelets were separated by Ku...
Embodiment 2
[0039] A11 inhibits platelet clot retraction.
[0040] (1) The source of experimental human platelets is the same as that of Example 1.
[0041] (2) The specific implementation is as follows:
[0042] Platelet-rich plasma was diluted with Tyrode's Buffer A and quantified to 500 × 10 9 / L, take 200 μL of platelet-rich plasma into a siliconized transparent glass tube, and incubate at 37°C for 20 minutes. A11 was dissolved in DMSO to adjust the concentration to 200 mM. The experimental groups were: positive control group (Thrombin 0.2U / mL), negative control group (Control) and A11 experimental group. A11 with a final concentration of 1 mM was added to the experimental group, the same volume of DMSO was added to the positive control group and the negative control group as the experimental group, and incubated at 37°C for 20 minutes. After incubation, add thrombin with a final concentration of 0.2 U / mL to the positive control group and A11 experimental tube, mix well, and place...
Embodiment 3
[0045] Take the polypeptide A11, dissolve it with a small amount of DMSO, add water for injection as usual, finely filter it, and fill it with sterilization to prepare an injection solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com