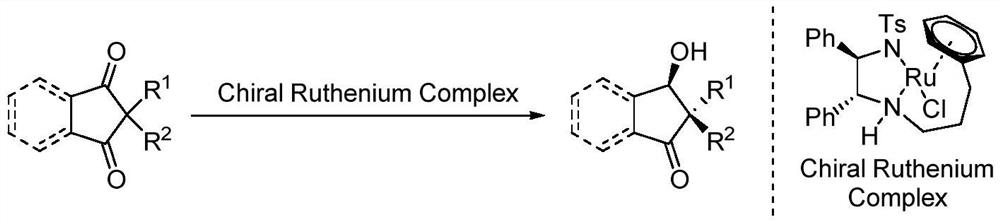
Method for synthesizing cis-beta-hydroxy ketone through ruthenium catalytic transfer hydrogenation desymmetry
A technology of transfer hydrogenation and desymmetry, applied in organic chemistry methods, chemical instruments and methods, formation/introduction of hydroxyl groups, etc., can solve problems such as limited examples
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-8
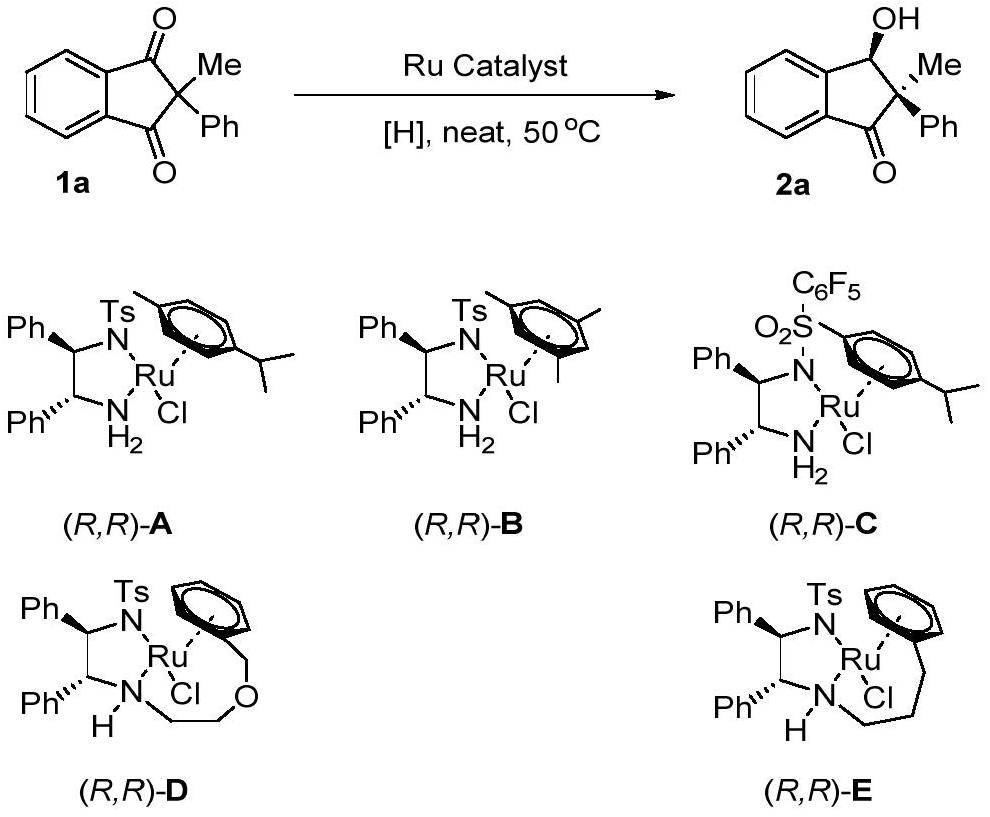
[0023] Optimization of hydrogenation reaction conditions
[0024] Under nitrogen atmosphere, drop into the chiral diamine complex catalyst of ruthenium (5mol% of substrate consumption) formic acid / triethylamine azeotrope (HCO 2 H / Et 3 N, the molar ratio of formic acid and triethylamine is 5:2), stirred and reacted at 50°C for 6 hours. Then, stop the reaction, add aqueous solution, extract with EA, combine the organic layers, remove the extraction solvent under reduced pressure, and separate by column chromatography to obtain pure product. The reaction formula and catalyst structure are as follows:
[0025]
[0026] The yield is the conversion rate, and the enantiomeric excess of the product is determined by chiral liquid chromatography, and the type of organic solvent in the process is changed, see Table 1 for details.
[0027] Table 1. Optimization of desymmetrization conditions for 1,3-diketone hydrogenation a
[0028]
[0029]
Embodiment 9-23
[0031] 2,2-Disubstituted-1,3-cyclopentanedione 1 Hydrogenation Desymmetry Synthesis of cis β-Hydroxyketone Derivative 2
[0032] Under nitrogen atmosphere, drop into the chiral diamine complex catalyst of ruthenium (5mol% of substrate consumption) and formic acid / triethylamine azeotrope 1.00mL (HCO 2 H / Et 3 N, the molar ratio of formic acid and triethylamine is 5:2), stirred and reacted at 50°C for 6 hours. Then, stop the reaction, remove the solvent under reduced pressure, add water, extract with EA, combine the organic layers, remove the solvent under reduced pressure, separate the pure product by column chromatography, change the type of reaction substrate, see the reaction formula for specific reaction conditions and parameters And Table 2, the reaction formula is as follows:
[0033]
[0034] Table 2.1,3-diketone hydrogenation desymmetrization substrate expansion
[0035]
[0036]
[0037] [a]Conditions:1(0.25mmol),(R,R)-Teth-Tsdpen-RuCl(5mol%),HCO 2 H / Et 3N...
Embodiment 24-29
[0040] 2,2-Disubstituted-1,3-cyclopentanedione 1 Hydrogenation Desymmetry Synthesis of cis β-Hydroxyketone Derivative 2
[0041] Under nitrogen atmosphere, drop into the chiral diamine complex catalyst of ruthenium (5mol% of substrate consumption) and formic acid / triethylamine azeotrope 1.00mL (HCO 2 H / Et 3 N, the molar ratio of formic acid and triethylamine is 5:2), stirred and reacted at 50°C for 6 hours. Then, stop the reaction, remove the solvent under reduced pressure, add water, extract with EA, combine the organic layers, remove the solvent under reduced pressure, separate the pure product by column chromatography, change the type of reaction substrate, see the reaction formula for specific reaction conditions and parameters , the reaction is as follows
[0042]
[0043] [a] Protected with p-bromobenzoyl chloride.
[0044] The yield is an isolated yield, and the enantiomeric excess of the product is determined by chiral liquid chromatography.
[0045] (+)-(2R,3R)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com