Preparation method and application of compound
A technology of a drug and ethoxyphenoxy, applied in the field of medicine, can solve problems such as no reports on synthesis methods and pharmacological effects, no reports on SphK1 inhibitors in literature, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
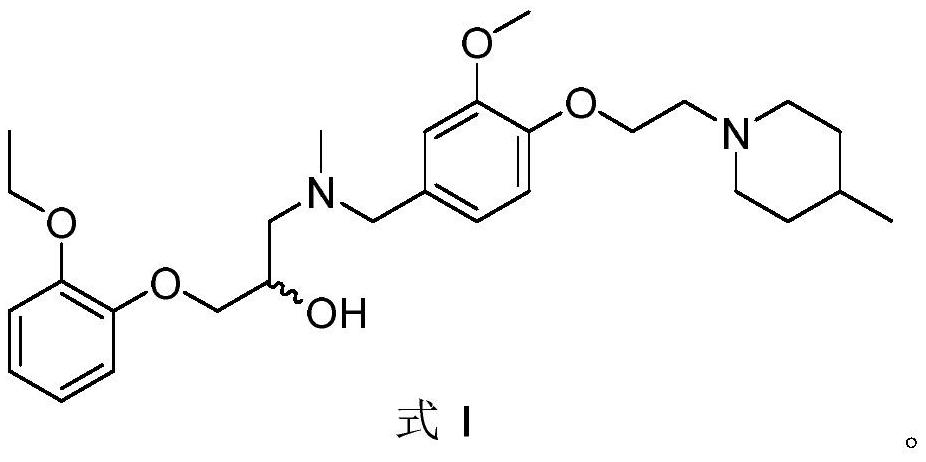
[0028] Example 1: 1-(2-ethoxyphenoxy)-3-((3-methoxy-4-(2-(4-methylpiperidin-1-yl)ethoxy)benzyl ) (methyl) amino) propan-2-ol (SAMS10) synthesis
[0029]
[0030] 2-((2-ethoxyphenoxy)methyl)oxirane (194mg, 1.0mmol) and 1-(3-methoxy-4-(2-(4-methylpiperidine- 1-yl)ethoxy)phenyl)-N-methylmethylamine (354mg, 1.2mmol) was dissolved in isopropanol (15mL), under nitrogen protection, a catalytic amount of pyridine (8.0μL, 0.1mmol) was added , heated to reflux for 6h, TLC detection (developing solvent: dichloromethane-methanol = 10:1) disappearance of raw materials. The reaction solution was diluted with ethyl acetate, the organic phase was washed with water and saturated brine successively, dried over anhydrous sodium sulfate, filtered, and the solvent was distilled off under reduced pressure. The crude product was separated and purified by silica gel column chromatography (mobile phase: dichloromethane-methanol=20 :1), a colorless oil (437.9mg, 90%) was obtained. 1 H NMR (CDCl ...
Embodiment 2
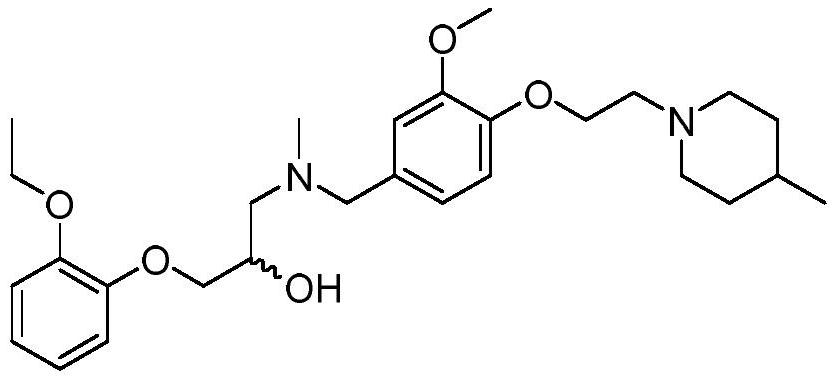
[0031] Example 2 1-(2-ethoxyphenoxy)-3-((3-methoxy-4-(2-(4-methylpiperidin-1-yl)ethoxy)benzyl) (Methyl)amino)propan-2-ol (abbreviated as SAMS10 in the experiment) for the antagonistic activity of SphK1 and SphK2 kinases
[0032] 1. Experimental instruments and materials
[0033] The multifunctional microplate reader used in the experiment is the SpectraMax M5 multifunctional microplate reader produced by Molecular Devices, USA.
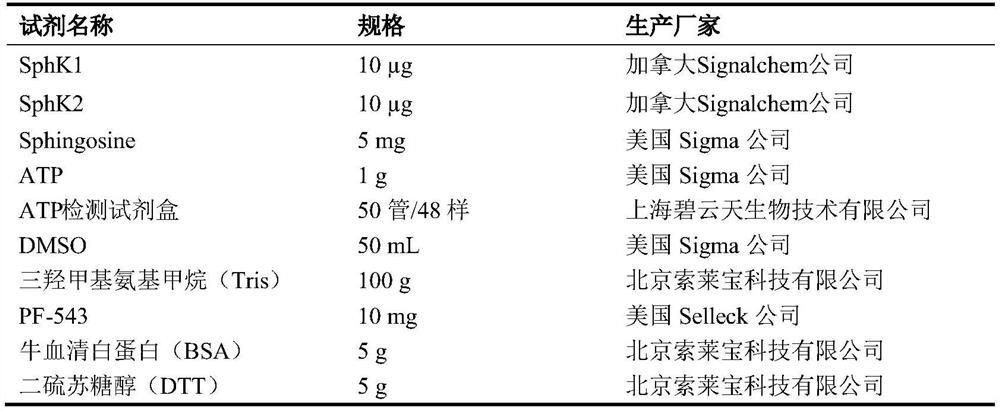
[0034] Table 1 Experimental reagents
[0035]
[0036] 2. Experimental method
[0037] First, 1-(2-ethoxyphenoxy)-3-((3-methoxy-4-(2-(4-methylpiperidin-1-yl)ethoxy)benzyl)( Methyl)amino)propan-2-ol was prepared into 10mM mother solution with DMSO, dissolved by ultrasonic acceleration, and then DMSO and kinase buffer (pH 7.4, 40mM Tris, 10mM MgCl 2 , 0.1g / L BSA, 1mM DTT, 10μM ATP) were serially diluted, and the final concentration of DMSO was less than 1%. The concentration in the initial screening was set to 10 μM, and the IC was determined 5...
Embodiment 3
[0051] Example 3 1-(2-ethoxyphenoxy)-3-((3-methoxy-4-(2-(4-methylpiperidin-1-yl)ethoxy)benzyl) (Methyl)amino)propan-2-ol (referred to as SAMS10 in the experiment) to test the growth inhibitory activity of tumor cells
[0052] 1. Experimental instruments and materials
[0053] The multifunctional marker analyzer used in the experiment is Victor 1420 model produced by Perkin Elmer Company of the United States.
[0054] Table 4 Experimental Reagents
[0055]
[0056] Cell lines: human lung cancer (A549), human ovarian cancer (SKOV3), human melanoma (A375) and human colon cancer (LOVO) cell lines, all purchased from the Cell Bank of the Chinese Academy of Sciences, using DMEM containing 15% fetal bovine serum ( High Glucose) medium, placed at 37 ° C, 5% CO 2 Cultured in an incubator.
[0057] 2. Experimental method
[0058] 2.1 MTT experimental principle and preparation method
[0059] MTT is an oxidative yellow dye with a chemical name of 3-(4,5-dimethyl-2-thiazolyl)-2,5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com