Synthesis method of drospirenone
A synthetic method, the technology of drospirenone, applied in the field of organic synthesis, can solve problems such as side reactions, increased production costs, and expensive raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
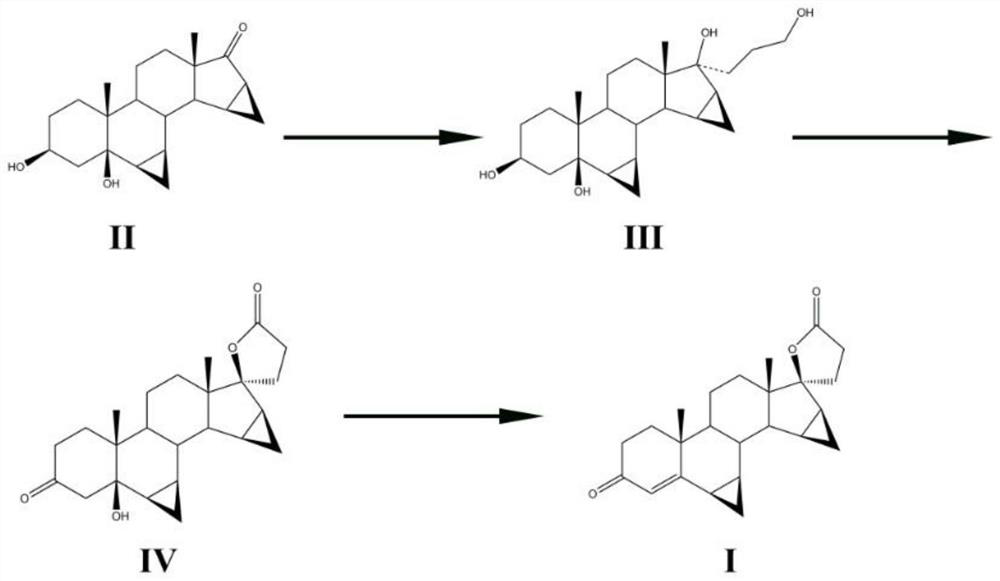
[0025] The invention provides a kind of synthetic method of drospirenone, comprising the following steps:
[0026] (1) metal lithium, 3-bromopropyl methyl ether and an aprotic organic solvent are mixed for an addition reaction to obtain an organolithium reagent;
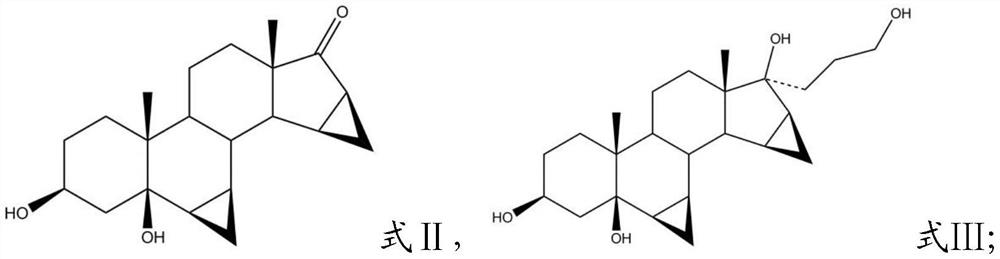
[0027] (2) The organolithium reagent obtained in the step (1), the compound having the structure shown in formula II, and an aprotic organic solvent are mixed for a nucleophilic substitution reaction, and then mixed with an organic acid and water for a hydrolysis reaction to obtain A compound of the structure shown in formula III;
[0028]
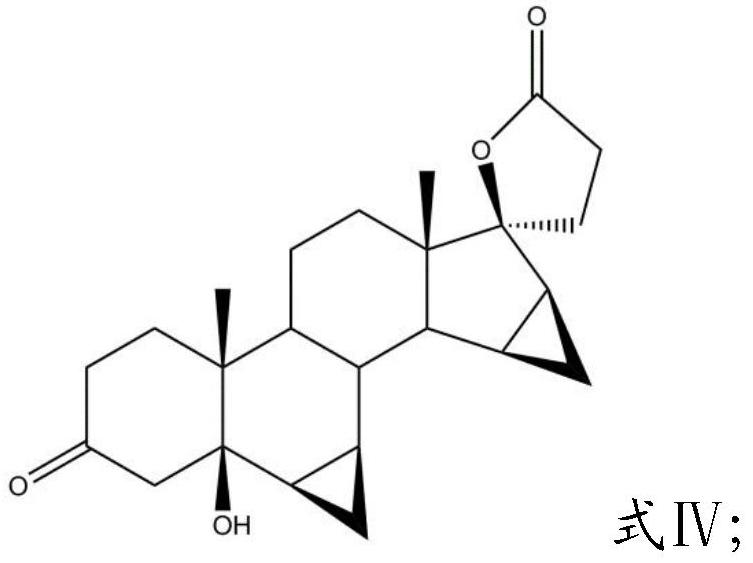
[0029] (3) Mix the compound having the structure shown in the formula III obtained in the step (2) with a noble metal catalyst, persulfate, water and an aprotic organic solvent, and carry out oxidation reaction and lactonization reaction in sequence to obtain the compound having the formula IV Compounds of the structure shown;
[0030]
[0031] (4) Mixing the compound hav...
Embodiment 1
[0077] The synthetic method of drospirenone:
[0078] (1) Add 100mL of anhydrous tetrahydrofuran (THF) and 40mL of 3-bromopropyl methyl ether into a three-necked flask, pass through nitrogen for protection, and cool the reaction system to 0°C. Lithium metal, to avoid liquid splashing caused by too violent reaction, and then carry out addition reaction at 0°C for 1 h with stirring to obtain an organolithium reagent solution.
[0079] (2) The organolithium reagent solution obtained in (1), 10 g of 3β, 5β-dihydroxy-6β, 7β, 15β, 16β-dimethylene-pregnant-17-one (formula II) Compound with the structure shown) and 100mL of THF were mixed, and then the organolithium reagent solution obtained in the step (1) was added dropwise to the reaction system. After the addition was completed, the nucleophilic substitution reaction was carried out under stirring at room temperature for 5 to 8 hours. After TLC monitors that the reaction is completed, the reaction mixture solution is poured into ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com