A kind of antibody-coupled drug and its application
An antibody and coupling technology, applied in the field of biopharmaceuticals, can solve the problems of weak immune effect and weak CDC effect, and achieve the effects of good uniformity, broadening research ideas, and good tumor killing ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
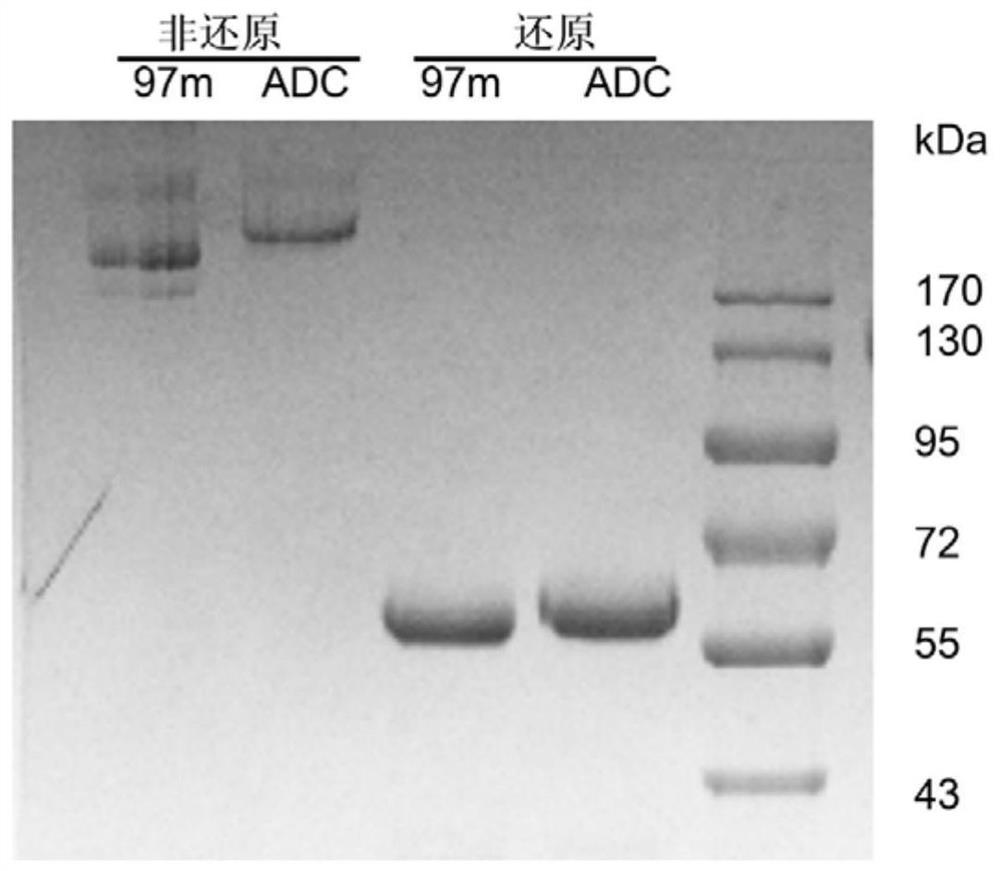
[0039] Preparation of 97m-vcMMAE conjugates.
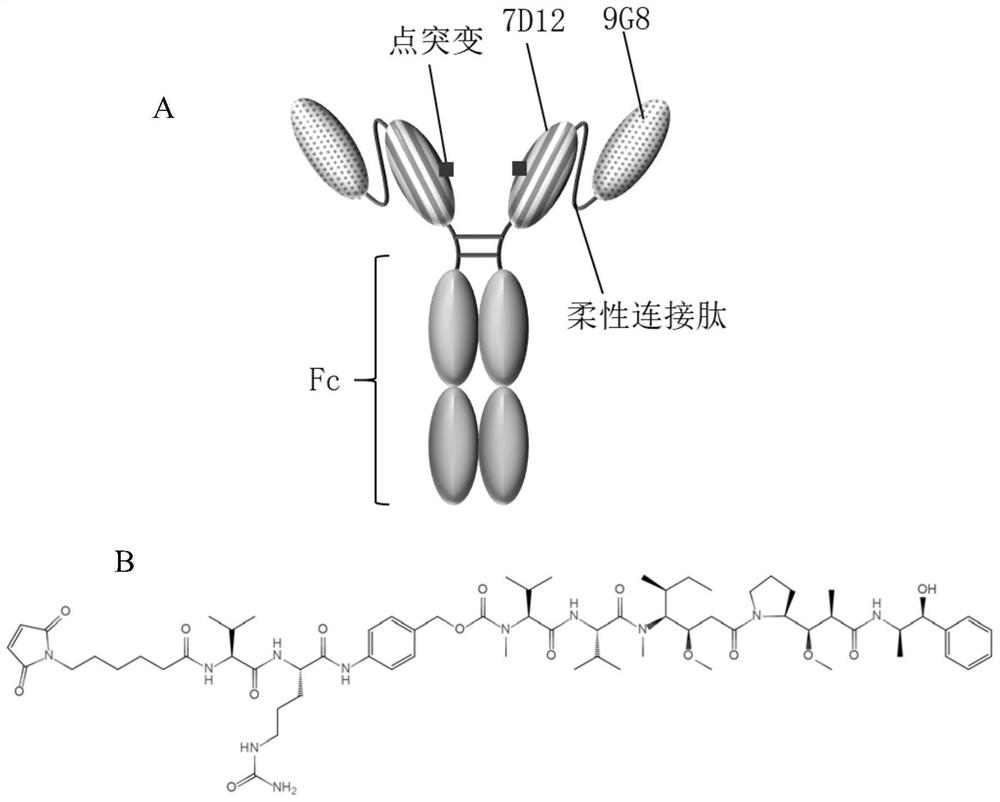
[0040] Nanobody Fc fusion protein targeting EGFR, 7D12 and 9G8 nanobody fusion, the sequence of the nanobody is from the literature (Karl R.Schmitz et al., Structure.2013; 21(7):1214–1224), the following It is called 97m for short. The Fc segment sequence of the fusion protein is preserved in the laboratory. In this embodiment, 7D12 and 9G8 were passed through the flexible linker peptide (GGGGS) by PCR method 3 The C-terminus is connected to the IgG1 heavy chain constant region, and finally the DNA sequence is obtained.
[0041] The sequence is as follows:
[0042] 9G8: the amino acid sequence is shown in SEQ ID No.1, and the gene sequence is shown in SEQ ID No.2.
[0043] 7D12: the amino acid sequence is shown in SEQ ID No.3, and the gene sequence is shown in SEQ ID No.4.
[0044] Flexible connecting peptide: the amino acid sequence is shown in SEQ ID No.5, and the gene sequence is shown in SEQ ID No.6.
[0045] IgG1 type h...
Embodiment 2
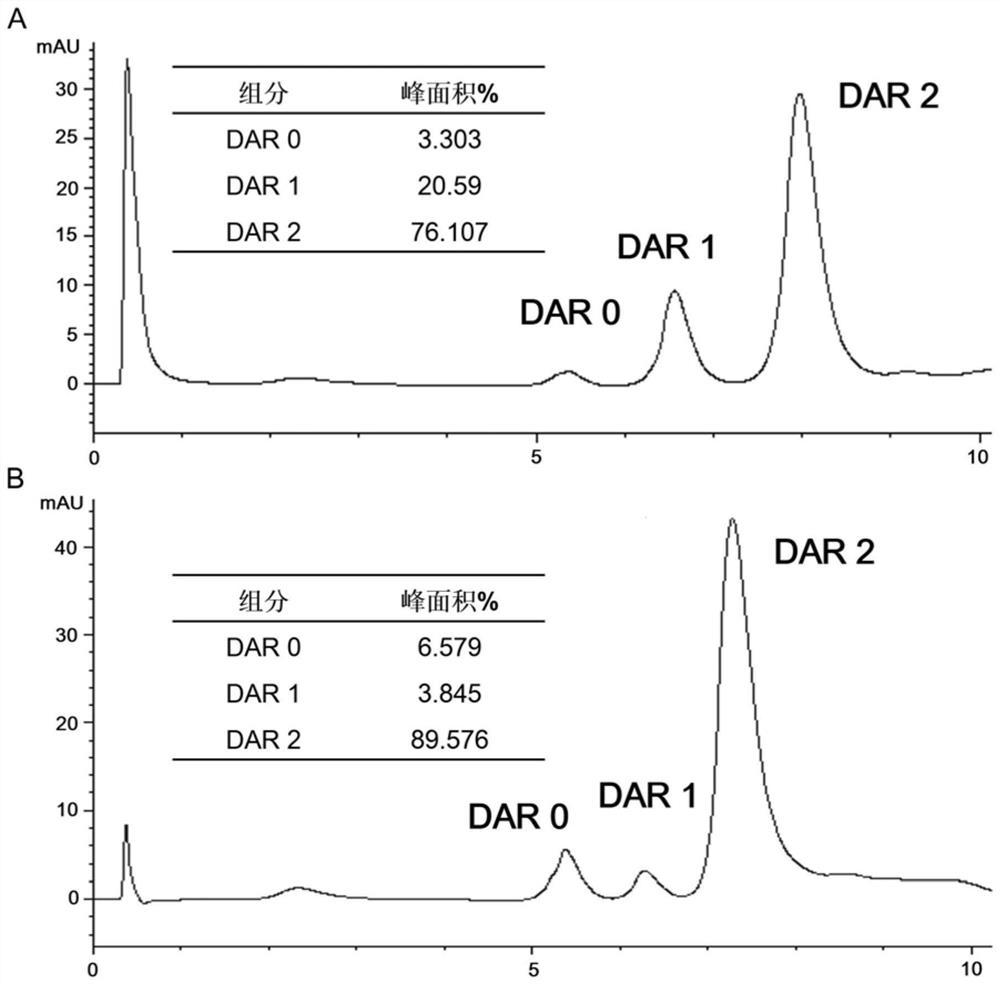
[0051] Drug-antibody conjugation ratio determination of conjugates.
[0052] In order to quantitatively detect the DAR value after coupling toxic small molecules, the coupled products were analyzed by hydrophobic interaction chromatography. The ADC concentration after coupling was diluted to 1 mg / mL before injection. Due to the strong hydrophobicity of vcMMAE small molecules, the changes in hydrophobicity after coupling small molecules can be used to distinguish the components of different drug small molecule coupling ratios in the final coupling product. The specific analysis conditions are as follows:
[0053] Chromatographic column: TSKgel Butyl-NPR hydrophobic chromatographic column (2.5μm, 4.6mm×3.5cm); mobile phase A: 2M (NH 4 ) 2 SO 4 , 150mM Na 3 PO 4 , pH 7.0; mobile phase B: 150mM Na 3 PO 4 , pH 7.0; mobile phase C: isopropanol; flow rate: 0.8mL / min; column temperature: 25°C; injection volume: 10μL;
[0054] Table 1
[0055] time (min) A% B% C% ...
Embodiment 3
[0059] Flow cytometry affinity assay.
[0060] Cells expressing EGFR, such as A431, were cultured with DMEM medium containing double antibodies. After the cells grew to the logarithmic growth phase, the cells were divided into 5×10 5 The number of cells was co-incubated with a series of serially diluted antibodies or ADCs in 1% BSA in PBS for 30 min on ice.
[0061] After the incubation, the cells were washed twice with pre-cooled PBS to remove proteins non-specifically adsorbed to the cell surface. Finally, a 1:200 dilution of FITC-labeled goat anti-human IgG secondary antibody was added in a dark environment, and the incubation was continued on ice for 30 min in the dark. After the incubation, the cells were washed with pre-cooled PBS, and the cell suspension resuspended in PBS was loaded on a flow cytometer to detect the mean fluorescence intensity (MFI) of the cells.
[0062] The affinity of the fusion protein to EGFR-positive cells before and after coupling was detected...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com