Stable isotope amino compound labeling reagent as well as synthesis method and application thereof
An amino compound and stable isotope technology, applied in the direction of isotope introduction into organic compounds, organic chemistry methods, chemical instruments and methods, etc., can solve the problems of poor labeling accuracy and sensitivity, difficult to obtain isotope products, complex synthesis of labeling reagents, etc. The effect of high labeling yield, simple detection of amino compounds, and fast labeling reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
no. 1 example
[0050] Refer to the following route 1 (wherein, X=D) to carry out the heavy-duty labeling reagent [d 4 The synthesis of ]-2-(9-acridone) ethyl chloroformate comprises the steps:
[0051] 1. Intermediate I: [d 5 Preparation of ]-N-phenylanthranilic acid:
[0052] Add 20g of anthranilic acid, 1g of copper powder and 10g of potassium carbonate to a 250mL three-necked flask, then add 150mL of dimethyl sulfoxide as a solvent, stir and mix evenly, then add 15g of deuterated bromobenzene- d 5 , and then reacted with stirring in an oil bath at 150° C. for 5 hours (anthranilic acid and deuterated bromobenzene-d 5 undergo a substitution reaction).
[0053] After the reaction, filter, remove excess copper powder and potassium carbonate, and collect the filtrate. Pour the filtrate into 200mL of clear water, adjust the pH value to 5 with concentrated hydrochloric acid, stir thoroughly, filter after the solid product is completely precipitated, put the obtained solid product in an oven...
no. 2 example
[0072] The reference application number is] CN202010117911.8, the synthetic route in the Chinese patent literature titled "Acridone Fluorescamine Compound Labeling Reagent and Its Synthetic Method and Application", to prepare light labeling reagent [d 0 ]-2-(9-acridone) ethyl chloroformate, the target product B.
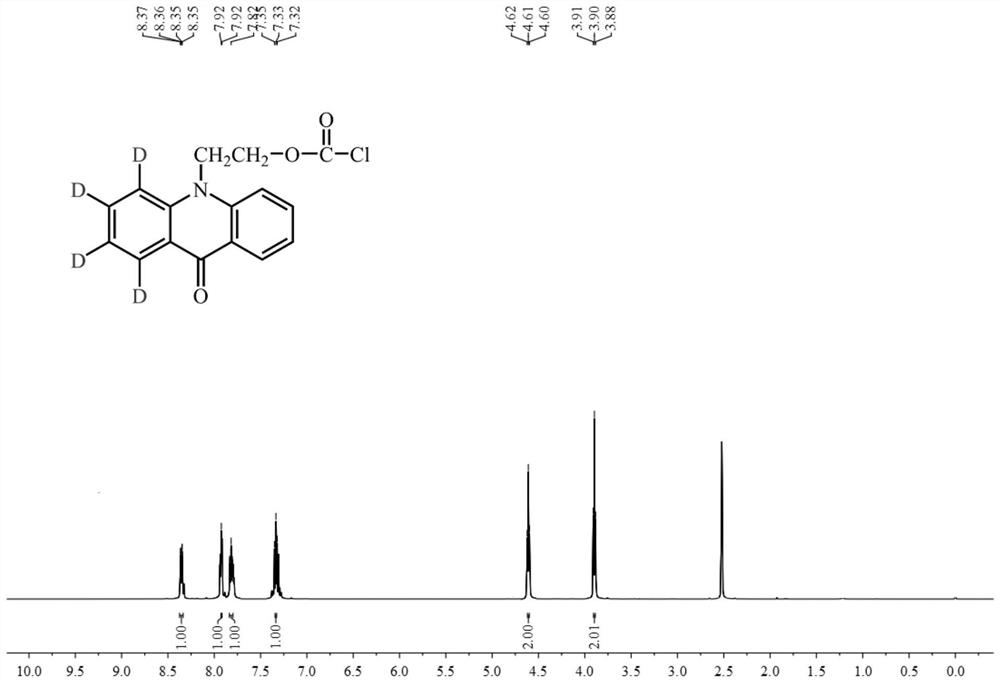
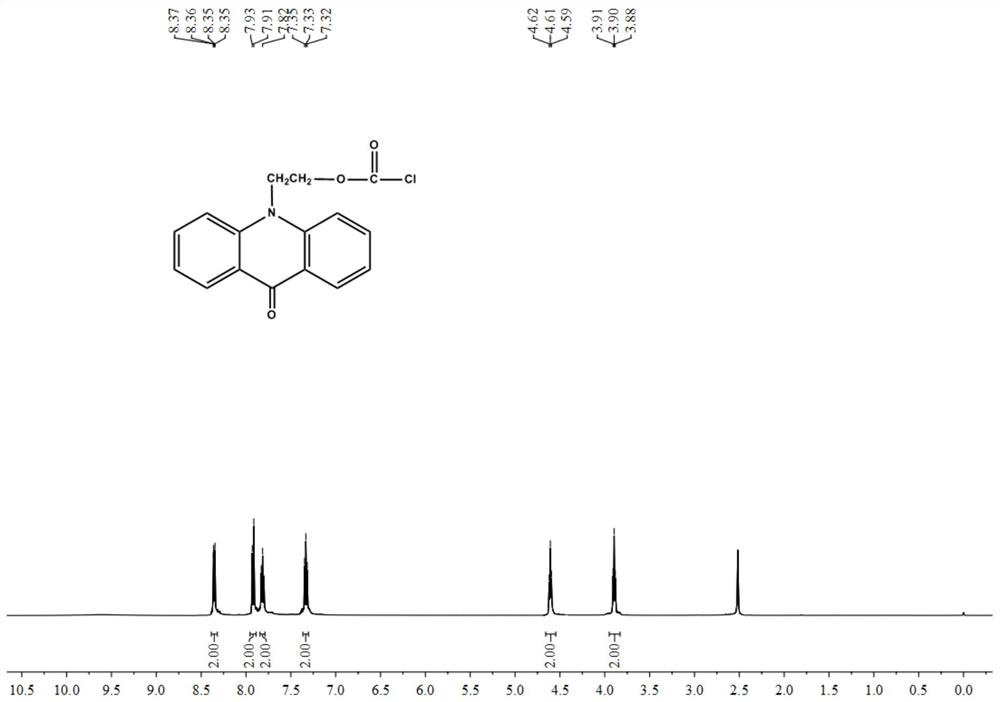
[0073] The target product B synthesized in this embodiment is characterized, and its NMR 1 H NMR spectrum such as figure 2 Shown:
[0074] 1 HNMR (500MHz, DMSO) δ8.36 (dd, J = 8.0, 1.5Hz, 2H), 7.92 (d, J = 8.8Hz, 2H), 7.82 (ddd, J = 8.7, 7.0, 1.6Hz, 2H), 7.33(t, J=7.4Hz, 2H), 4.61(t, J=6.3Hz, 2H), 3.90(t, J=6.3Hz, 2H).
[0075] 13 CNMR (126MHz, DMSO) δ176.96, 142.44, 142.32, 134.39, 126.99, 122.02, 121.65, 116.88, 58.59, 48.03
[0076] Found: Found: C63.79, H3.99Cl11.79, N4.65, O15.95; Calculated: C63.69, H4.01Cl11.75, N4.64, O15.91.
[0077] MS:m / z:[M+H] + = 302.1.
[0078] It can be seen that the target product B was successfully synthesized in this examp...
no. 3 example
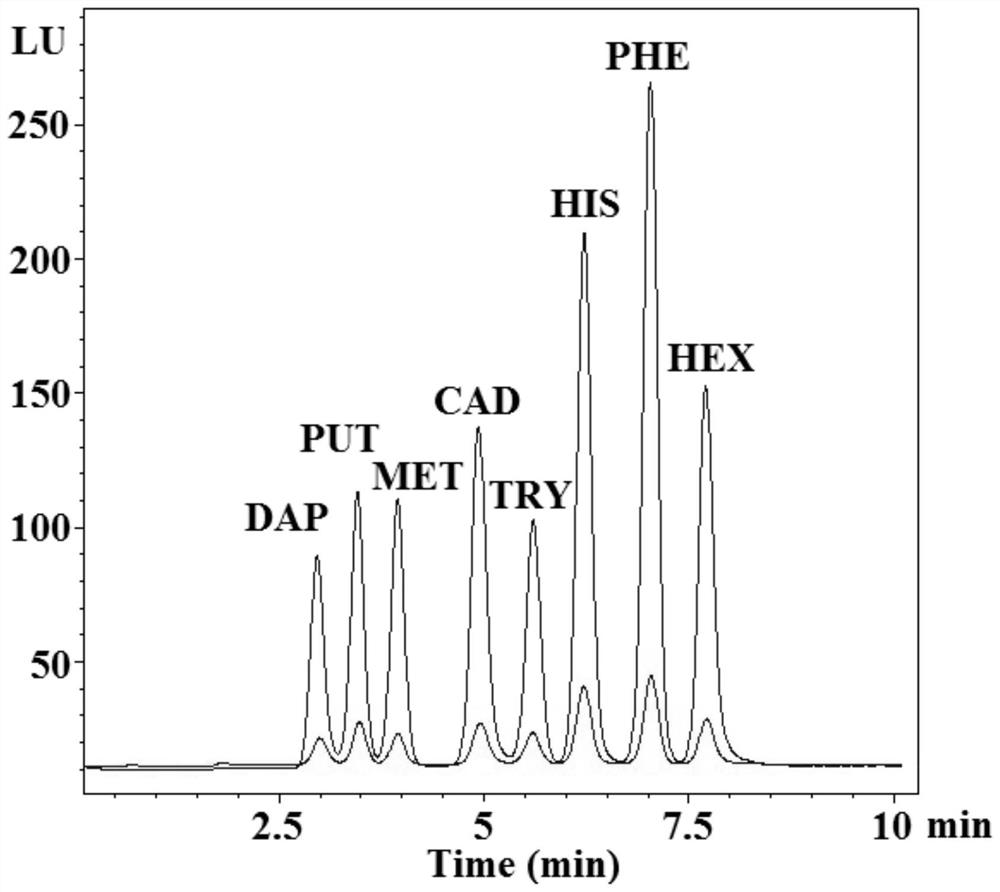
[0081] A method for detecting the concentration of biogenic amine in an analyte using a stable isotope amino compound labeling reagent, that is: using the heavy-duty labeling reagent and light-duty labeling reagent prepared respectively in the first embodiment and the second embodiment to test biogenic amine, details as follows:
[0082] The first step: prepare borax / boric acid buffer solution with pH=9.5; the preparation concentration is 1×10 -6 mol / L biogenic amine standard acetonitrile solution; the prepared concentration is 1×10 -4 mol / L[d 0 ]-2-(9-acridone) ethyl chloroformate in acetonitrile solution as a light labeling reagent solution; the preparation concentration is 1×10 -4 mol / L[d 4 ]-2-(9-acridone) ethyl chloroformate in acetonitrile solution as a heavy-duty labeling reagent solution for subsequent use.
[0083] Step 2: sequentially add 200 μL of the borax / boric acid buffer solution, 100 μL of biogenic amine standard solution and 50 μL of light labeling reagent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com