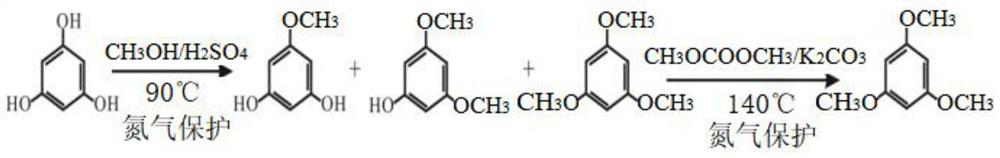
Preparation method of trimethylphloroglucinol
A technology of trimethylphloroglucinol and anhydrous phloroglucinol, which is applied in the field of medicine, can solve the problems of high product yield, reagent toxicity, and low safety, and achieve less by-products, easy operation, and improved safety sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] The present embodiment provides a kind of preparation method of trimethylphloroglucinol, comprising the following steps:
[0034] (1) Take 200ml of methanol and add it to the reaction bottle, slowly drop 64g of concentrated sulfuric acid under stirring; take 20.2g of anhydrous phloroglucinol into the reactor, stir until dissolved, fill with nitrogen, and then heat to 80°C for 6h under reflux .
[0035] Cool to room temperature, add 200ml of 20% sodium chloride solution, extract with methyl tert-butyl ether (100ml+50ml+50ml), combine the extracts, and distill at 60°C to recover the solvent to obtain a concentrate.
[0036] (2) Take the concentrate of step (1), add 180ml of dimethyl sulfoxide to dissolve, fill with nitrogen, add 66.4g of potassium carbonate, and raise the temperature to 130°C. Weigh 57.8g of dimethyl carbonate, divide it into three additions, each interval is 0.5h, continue to react for 1h after the addition, and then carry out distillation to remove unr...
Embodiment 2
[0040] The present embodiment provides a kind of preparation method of trimethylphloroglucinol, comprising the following steps:
[0041] (1) Take 200ml of methanol and add it to the reaction bottle, slowly drop 72g of 98% sulfuric acid solution under stirring; take 20.2g of anhydrous phloroglucinol into the reactor, stir until dissolved, fill with nitrogen, and then heat to 90°C to reflux Reaction 4h.
[0042] Cool to room temperature, add 200ml of 20% sodium chloride solution, extract with methyl tert-butyl ether (100ml+50ml+50ml), combine the extracts, and distill at 60°C to recover the solvent to obtain a concentrate.
[0043](2) Take the concentrate of step (1), add 180ml of dimethyl sulfoxide to dissolve until clear, fill with nitrogen, add 44.3g of potassium carbonate, and raise the temperature to 120°C. Weigh 72.2g of dimethyl carbonate, divide it into three additions, with an interval of 0.5h each time, continue to react for 2h after the addition, and then carry out d...
Embodiment 3
[0047] The present embodiment provides a kind of preparation method of trimethylphloroglucinol, comprising the following steps:
[0048] (1) Take 12L of methanol and add it to the reactor, slowly drop 3.84kg of 98% sulfuric acid solution under stirring; take 1.21kg of anhydrous phloroglucinol into the reactor, stir until dissolved, fill with nitrogen, and then heat to 90°C Reflux reaction 4h.
[0049] Cool to room temperature, add 12L of 20% sodium chloride solution, extract with methyl tert-butyl ether (6L+3L+3L), combine the extracts, and distill at 60°C to recover the solvent to obtain a concentrate.
[0050] (2) Take the concentrate of step (1), add 10.8 L of dimethyl sulfoxide to dissolve until clear, fill with nitrogen, add 3.98 kg of potassium carbonate, and raise the temperature to 140 °C. Weigh 4.33 kg of dimethyl carbonate, divide it into three additions, with an interval of 0.5 hours between each addition, continue to react for 2 hours, then carry out distillation ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com