In-vitro biological activity determination method of leech medicinal materials, decoction pieces and processed products
A technology of biological activity and measurement method, applied in the field of in vitro biological activity measurement, can solve the problems of weak correlation, undetectable antithrombin activity, weakened anticoagulant activity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
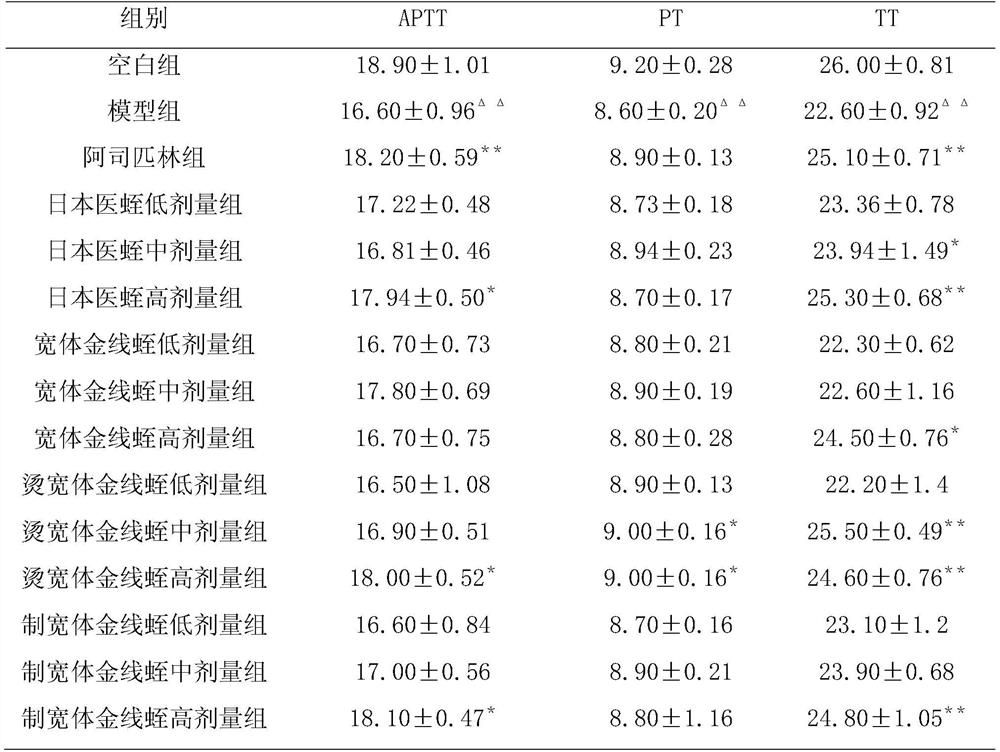
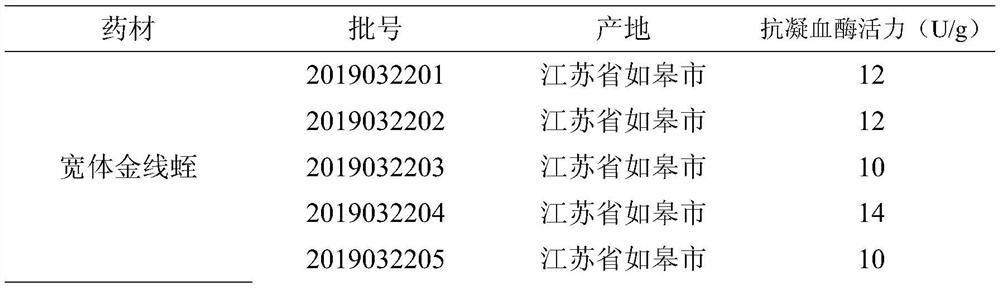
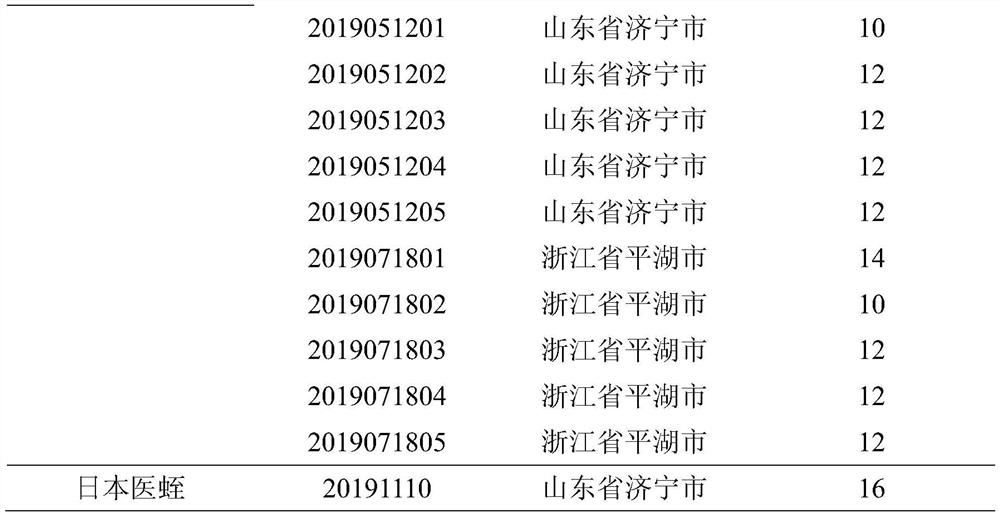
[0053] Determination of antithrombin activity of different batches of leeches decoction pieces: a total of 15 batches of leech were collected from 3 main producing areas of leeches in Rugao City, Jiangsu Province, Jining City, Shandong Province, and Pinghu City, Zhejiang Province herbs,
[0054] Preparation of the test solution: Take leech, crush it through a No. 3 sieve, weigh 2.5g, weigh it accurately, add 20ml of water, weigh it, heat at 85°C for 15 minutes, cool to 40°C, adjust the pH to 2.0, add pepsin with an enzyme-to-substrate ratio of 1% (enzyme activity is 1:15000), enzymolyze at 40°C for 1 hour, adjust the pH to 8.0 with 20% sodium hydroxide solution, add trypsin with an enzyme-to-substrate ratio of 1% (enzyme The activity is 2500U / mg), enzymolysis at 40°C for 3 hours, adding dilute hydrochloric acid to adjust the pH to near neutral, heating at 85°C for 15 minutes, cooling to room temperature, high-speed centrifugation, putting the supernatant in a 25ml measuring bo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Vitality | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com