Application of interferon lambda in treatment of novel coronavirus (2019-nCoV) infection
A technology for coronavirus and virus infection, applied in antiviral agents, medical preparations containing active ingredients, peptide/protein components, etc., can solve problems such as hematology and nervous system suppression side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Implementation example 1: Construction of interferon lambda expression engineering bacteria
[0048] Human interferon λ1 / λ2 / λ3 / λ4 gene fragments (NM_172140.2 / NM_172138.2 / NM_172139.2) were obtained by chemical synthesis, and inserted into the prokaryotic expression plasmid pET- 30a and verified by sequencing. Resulting expression plasmids for transformation assays.
[0049] The plasmid containing the target gene obtained above was transformed into BL21(DE3) competent cells (Invitrogen), 50 μl of BL21 competent cells were placed on an ice bath to melt, the target DNA was added, shaken gently, and placed in an ice bath for 30 minute. Then heat shock in a 42°C water bath for 30 seconds, and then quickly transfer the centrifuge tube to an ice bath for 2 minutes without shaking the centrifuge tube. Add 500 μl of sterile LB medium (without antibiotics) to the centrifuge tube, mix well, place at 37° C., and incubate at 180 rpm for 1 hour to recover the bacteria. Pipette 200...
Embodiment 2
[0050] Implementation example 2: expression and purification of interferon lambda
[0051] Add 50 μl of bacterial solution (bacterial solution expressing interferon lambda) to 50 ml of LB medium, and add 50 μl of kanamycin at the same time, mix well, place in a constant temperature shaker at 30°C, and inoculate overnight. Take 10ml of the overnight inoculated bacterial solution and add it to 1000ml LB medium, and add 1000μl kanamycin at the same time. Shake well and place in a shaker at 37°C at 200rpm, cultivate until the OD600 of the bacterial solution is 0.4-0.6, add 0.5mM IPTG for induction, continue to cultivate for 4 hours, and collect the bacterial cells. The expressed IL29 mutant accounts for about 30-50% of the total bacterial protein, and mainly exists in the form of inclusion bodies.
[0052] The fermented cells were washed with TE (10mmol / L Tris-HCl, 1mmol / L EDTA, pH 6.5) solution (m:V=1:10) for 3 times, then 60Mpa high-pressure homogenate was crushed, after homoge...
Embodiment 3
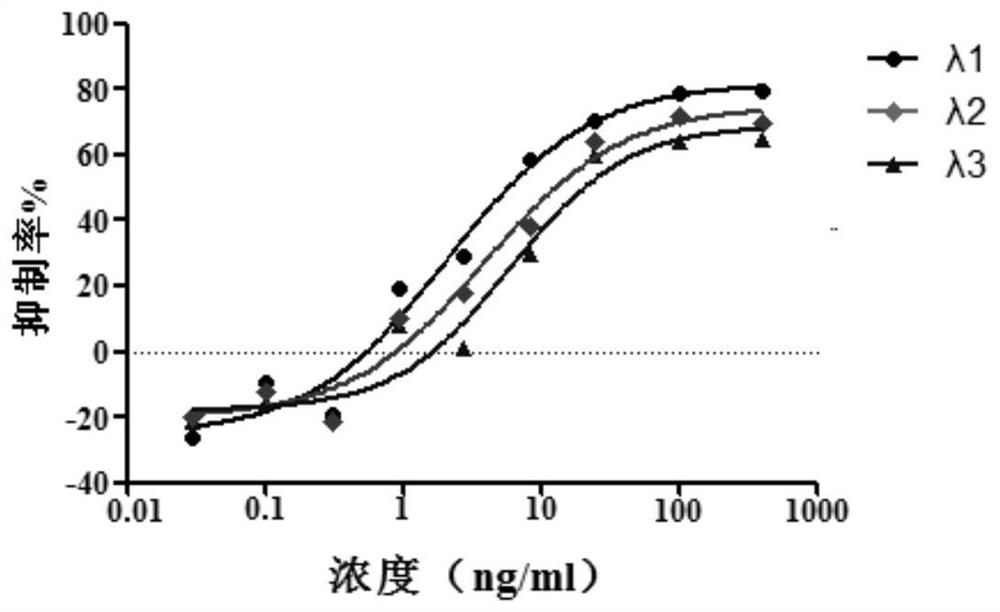
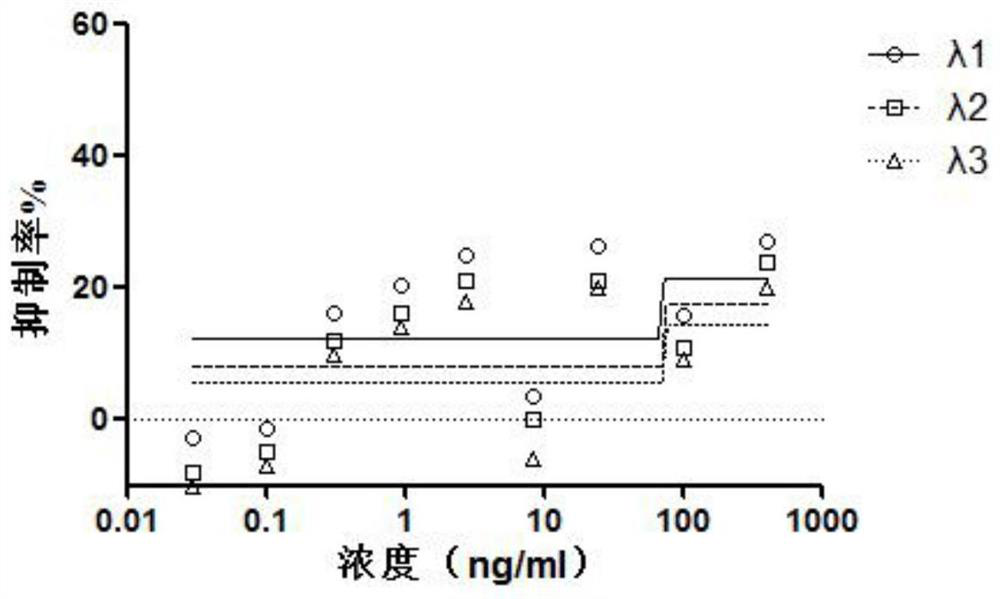
[0056] Example 3: In vitro drug efficacy of novel coronavirus
[0057] Cells: VeroE6 cells, preserved by the Pathogen Center, Institute of Medical Experimental Animals, Chinese Academy of Medical Sciences.
[0058] Virus: 2019-nCoV, titer 10 5 TCID 50 / ml, stored at -80°C by the Pathogen Center, Institute of Medical Experimental Animals, Chinese Academy of Medical Sciences. Use a viral titer of 100TCID 50 .
[0059] In a sterile 96-well culture plate, add 200 μl to each well with a concentration of 5×10 4 cell / ml Vero E6 cells, 37°C 5% CO 2 Cultivate for 24 hours;
[0060](2) The test drug was diluted to 2 concentrations, each concentration was replicated into 5 wells, and 100 μl was added to each well for 24 hours;
[0061] (3) Add 100 μl of 100 TCID to each well 50 Virus;
[0062] (4) Set up cell control, blank control (solvent control) and virus control (negative control) at the same time;
[0063] (5) Cells at 37°C, 5% CO 2 Incubation in the incubator for 4-5 da...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com