Heating synthesis method of uracil
A synthetic method and technology of uracil, which is applied in the field of uracil synthesis, can solve the problems of high equipment requirements, difficult separation of intermediates, and difficult availability of raw materials, and achieve the effects of good selectivity, low pollution, and easy liquid separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
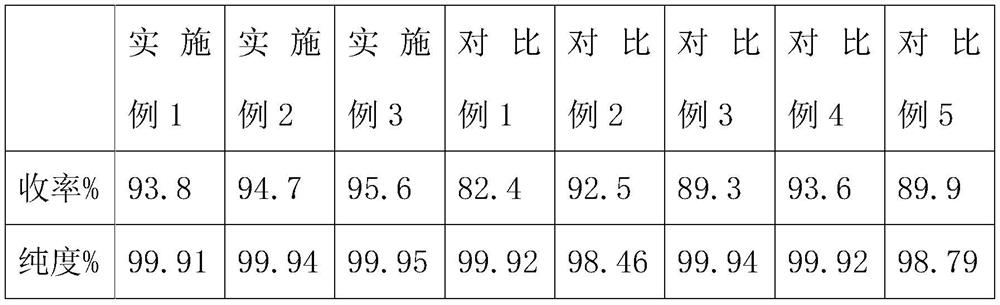
Embodiment 1
[0024] In embodiment 1, the thermal synthesis method of uracil comprises the following steps:
[0025] S1. Add an organic solvent, acetic anhydride and urea into the reaction vessel, and mix and react, wherein the reaction temperature is 25° C., and the reaction time is 2 hours;
[0026] S2, then add acetaldehyde and alkali to the reaction container, and add a catalyst to carry out condensation cyclization reaction, wherein the reaction temperature is 45 ° C, the reaction time is 2h, to obtain uracil;
[0027] In S1, the mol ratio of acetic anhydride and urea is 2.1:1, and described organic solvent is an inert organic solvent;
[0028] In S2, the mol ratio of acetaldehyde and urea is 1.3:1, and wherein alkali is ammoniacal liquor, and the mol ratio of ammonia in ammoniacal liquor and urea is 1.6:1, and described catalyzer is light microsphere catalyst, and catalyst add-on is urea quality 3%.
[0029] Further, the preparation method of the lightweight microsphere catalyst inc...
Embodiment 2
[0037] In embodiment 2, the thermal synthesis method of uracil comprises the following steps:
[0038] S1. Add an organic solvent, acetic anhydride and urea into the reaction vessel, and perform a mixed reaction, wherein the reaction temperature is 35° C., and the reaction time is 4 hours;
[0039] S2, then add acetaldehyde and alkali to the reaction vessel, and add a catalyst to carry out condensation cyclization reaction, wherein the reaction temperature is 65 ° C, the reaction time is h, to obtain uracil;
[0040] In S1, the mol ratio of acetic anhydride and urea is 2.4:1, and described organic solvent is an inert organic solvent;
[0041] In S2, the mol ratio of acetaldehyde and urea is 1.5:1, and wherein alkali is ammoniacal liquor, and the mol ratio of ammonia in ammoniacal liquor and urea is 1.9:1, and described catalyzer is light microsphere catalyst, and catalyst add-on is urea quality 5%.
[0042] Further, the preparation method of the lightweight microsphere catal...
Embodiment 3
[0050] In embodiment 3, the thermal synthesis method of uracil comprises the following steps:
[0051] S1. Add an organic solvent, acetic anhydride and urea into the reaction vessel, and perform a mixed reaction, wherein the reaction temperature is 30° C., and the reaction time is 3 hours;
[0052] S2, then add acetaldehyde and alkali to the reaction container, and add a catalyst to carry out condensation cyclization reaction, wherein the reaction temperature is 55 ° C, the reaction time is 4h, to obtain uracil;
[0053] In S1, the mol ratio of acetic anhydride and urea is 2.3:1, and described organic solvent is an inert organic solvent;
[0054] In S2, the mol ratio of acetaldehyde and urea is 1.4:1, and wherein alkali is ammoniacal liquor, and the mol ratio of ammonia in ammoniacal liquor and urea is 1.8:1, and described catalyzer is light microsphere catalyst, and catalyst add-on is urea quality 4%.
[0055] Further, the preparation method of the lightweight microsphere cat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com