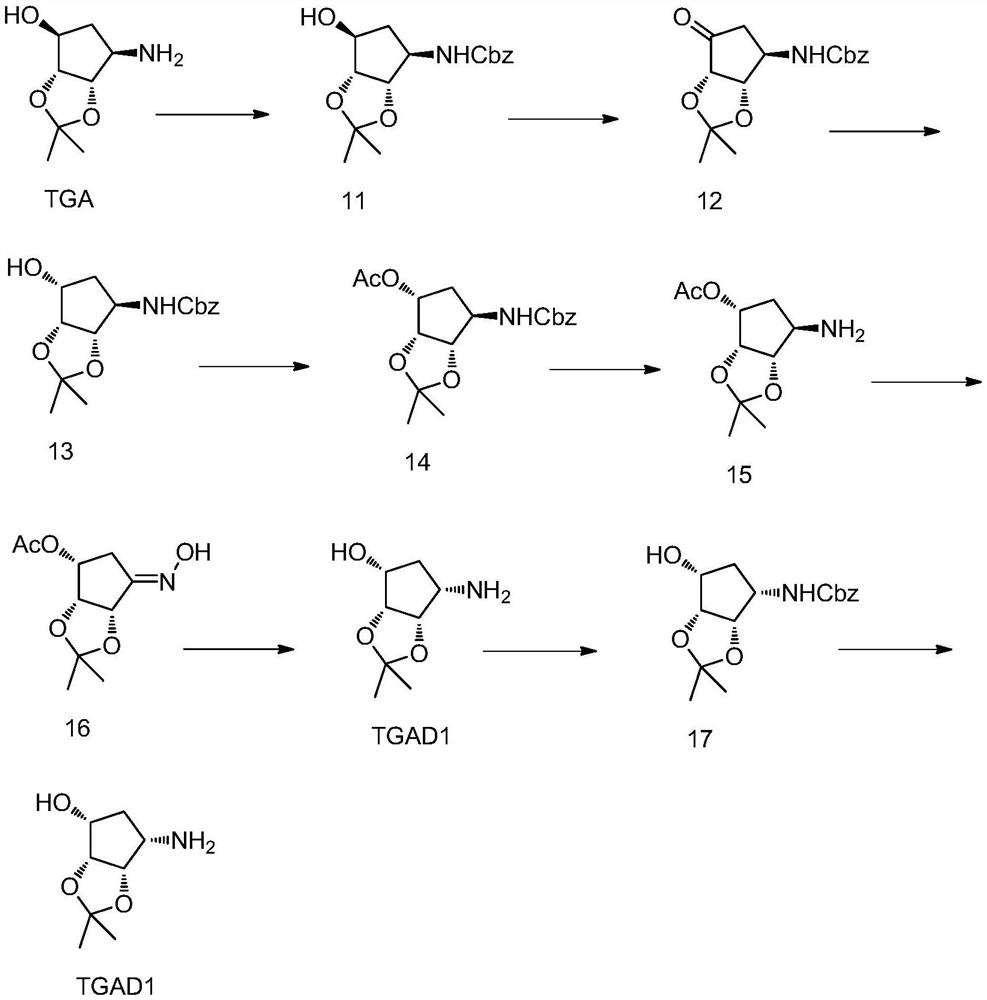
Preparation method of ticagrelor key chiral intermediate isomer impurity TGAD1
The technology of a chiral intermediate, ticagrelor, is applied in the field of medicinal chemistry to achieve the effects of mild reaction conditions, high refining yield and purity, and convenient post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Dissolve TGA (80 g, 0.4624 mol) in 250 mL of tetrahydrofuran, add 200 mL of water, and add potassium carbonate (127.62 g, 0.9248 mol). Stir to dissolve, lower the temperature to 0°C, add benzyl chloroformate (118.33g, 0.6936mol) dropwise, and stir at room temperature for 5min after dropping, the raw materials are completely converted, separate layers, add 150mL ethyl acetate to extract the aqueous layer, and combine the organic layers. Dried over anhydrous sodium sulfate, concentrated to obtain 110 g of product (HPLC: 95%);
[0035]
[0036] (2) Add the compound of formula 11 (100g, 0.3256mol), sodium bromide (16.75g, 0.1628mol), sodium bicarbonate (32.8g, 0.3905mol), TEMPO (763mg, 4.884mmol), into a 5000mL three-necked flask, Add 1L of dichloromethane and 1L of water, stir to dissolve. Cool down to 0°C, slowly add sodium hypochlorite solution (8%w, 800mL) dropwise, and control the temperature not to exceed 10°C. After dripping, keep warm for 10 minutes. TLC d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com