Preparation of self-assembled virus-like particle by using escherichia coli to express feline parvovirus VP2 protein
A feline parvovirus and parvovirus technology, applied to viruses, viral peptides, viruses/bacteriophages, etc., can solve problems such as research work and application obstacles, and low production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] The invention discloses a prokaryotic method for expressing feline parvovirus VP2 protein. In the method, the VP2 protein is co-expressed with a chaperone protein, and a recombinant expression protein with good solubility and high activity can be obtained. Its specific steps are as follows:
[0028] 1.1 Construction of recombinant vector pET30a-VP2
[0029] 1.1.1 Artificial modification and synthesis of FPV main immunogenic gene VP2
[0030] Referring to the FPV gene sequence, the sequence was optimized according to the codon preference of Escherichia coli, and the artificially synthesized complete feline parvovirus VP2 gene was inserted into the vector pUC to construct the plasmid pUC-VP2.
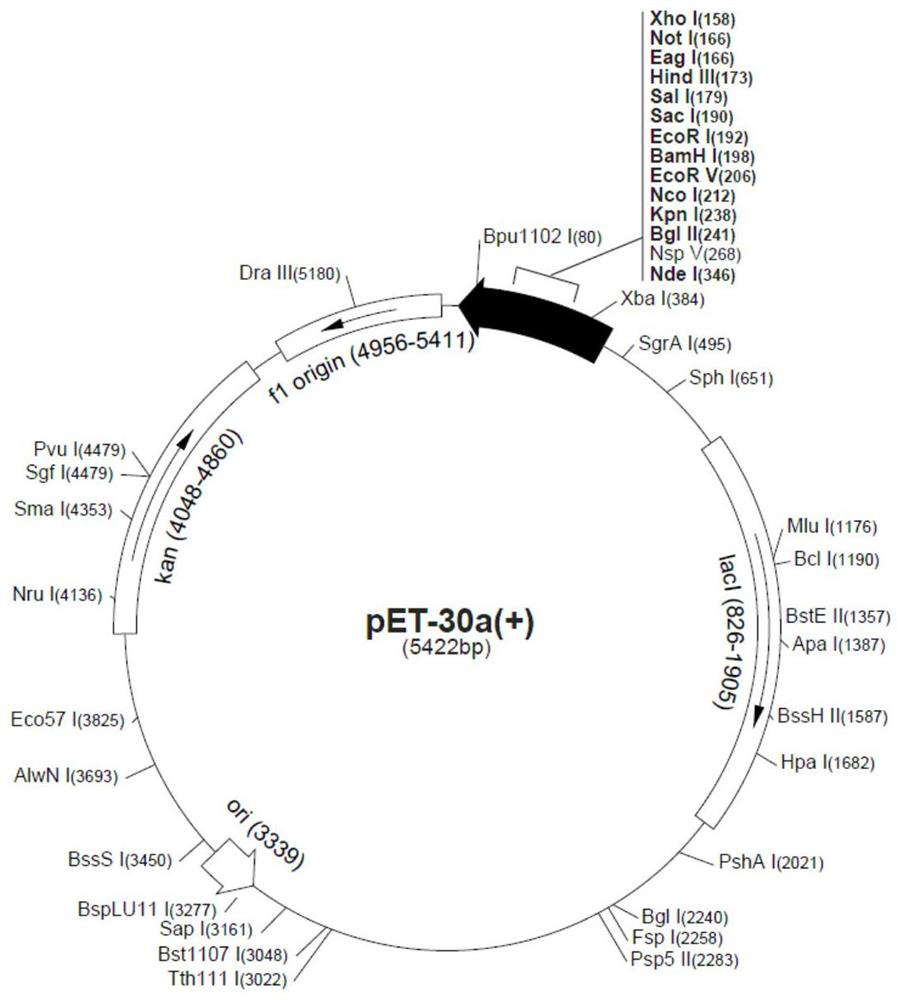
[0031] 1.1.2 Construction of pET30a-VP2 vector
[0032] Plasmid pUC-VP2 and vector pET30a were double-digested with restriction endonucleases NdeI and HindIII, respectively, and the reaction conditions were 37°C for 2 hours, and then the double-digested products of vector pET30a ...
Embodiment 2
[0044] Preparation of feline parvovirus virus-like particles
[0045]2.1 Purification of VP2 protein and determination of VLP
[0046] 2.1.1 Purification of recombinant VP2 protein
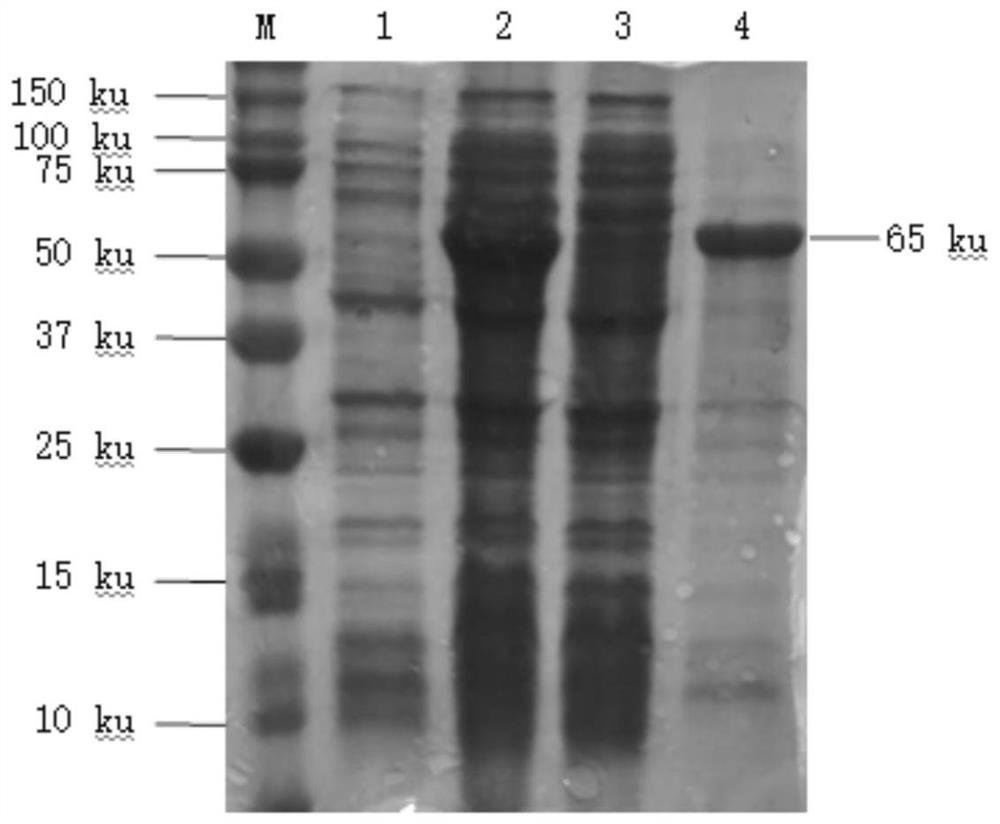
[0047] Add saturated ammonium sulfate solution to the supernatant of ultrasonic cracking obtained in Example 1, so that the final concentrations of ammonium sulfate are 15%, 20%, 25%, 30%, 35%, 40%, and 45% saturation respectively. Stir at 4°C for 1 h, centrifuge at 4000 rpm for 30 min, take the supernatant and resuspend the pellet with lysate (50 mM Tris, 150 mM NaCl, pH 8.0). After processing each supernatant and precipitation sample, SDS-PAGE detection, the results are as follows Image 6 , VP2 protein can remove a large number of foreign proteins after passing through 25% ammonium sulfate.
[0048] Add 25% ammonium sulfate precipitated protein to an equal volume of reconstituted solution (0.25M (NH4)2SO4, 20mM Tris, 2mMNaCl) to redissolve, use the reconstituted solution to wash the hydropho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com