Culture medium, induction culture method of oligodendrocyte progenitor cells and application
A culture method and culture medium technology, applied in the field of cell culture, can solve the problems of increased workload, time-consuming, tracking and testing of cell growth status, etc., and achieve the effects of shortening time, reducing biological pollution and physical pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1 Human oligodendrocyte progenitor cell hOPC induction production and culture method
[0080] 1) hESC or hiPSC cell lines were cultured in mTeSR1 medium on a six-well plate coated with vitronectin (VitronectinXF, 10 μg / ml / well). When the cell confluency reaches 90%, subculture or induce differentiation. Undifferentiated stem cells can be identified by ICC-verified expression of human pluripotent stem cell markers, such as SSEA4, TRA-1-60, OCT4, NANOG, and SOX2, or by qPCR-verified expression of genes such as OCT4, NANOG, SOX, and hTERT Identification. The average expansion time for each hESC or hiPSC line is 5-7 days.
[0081] 2) Generation of embryonic bodies (embryonic bodies, EBs): When hESC or hiPSC cells can be induced to differentiate, digest them with Dispase (1U / ml) at 37°C for 5-7min, and use a 5ml pipette to cut the cells into approx. Cell flakes of 2mm×2mm size. Collect all cell pieces, resuspend in 7ml of mTeSR1, and culture in T25 culture flask....
Embodiment 2
[0090] Example 2 hOPC induction production and verification test
[0091] 1) hESCs can efficiently and quickly induce hOPCs:
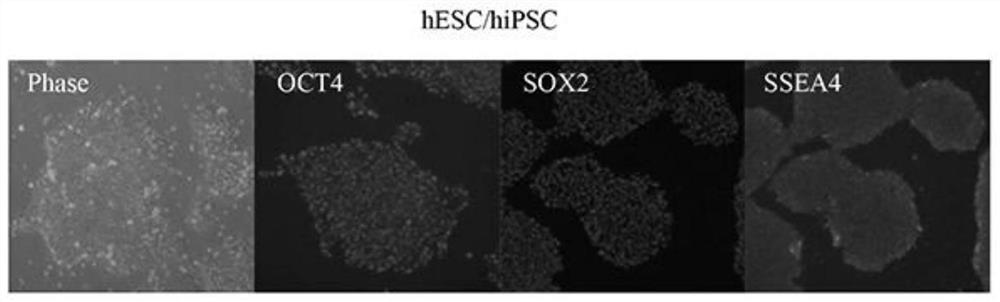
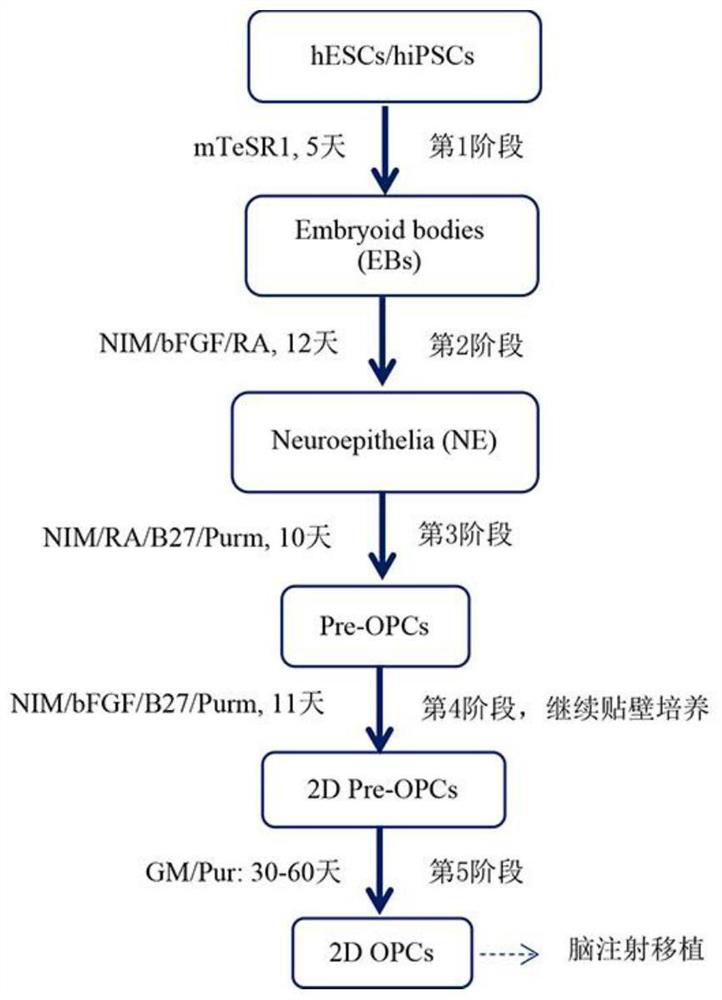
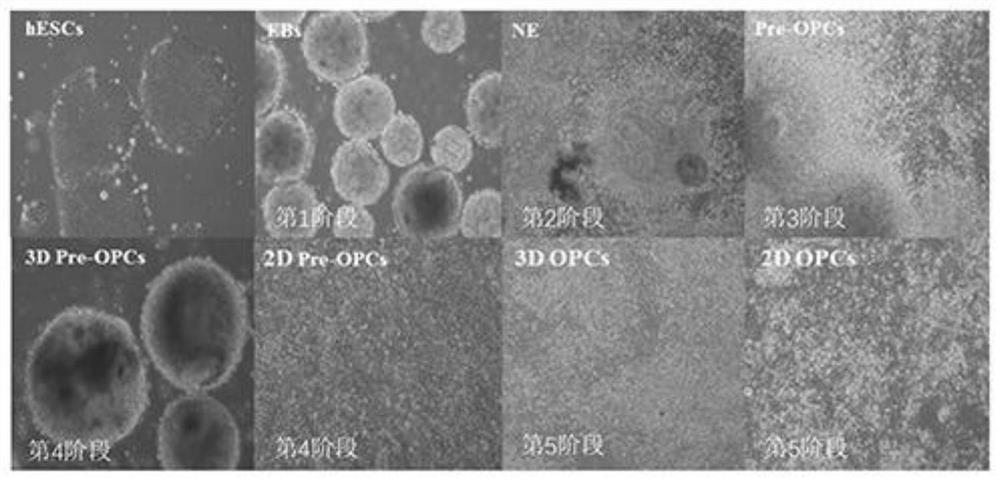
[0092] hESC cell lines, which can be confirmed by immunofluorescent staining (ICC) to express the characteristics of stem cell-specific markers SSEA4, OCT4 and SOX2, such as figure 1 shown. The culture program for the whole experimental process of effectively inducing hESCs to differentiate into hOPCs must go through 5 stages, and the in vitro culture takes 80-100 days ( Figure 2-3 ). mRNA was extracted from undifferentiated hESC and hOPC1 and hOPC2 induced to differentiate for 100 days for transcription and qPCR identification; qPCR results showed that in differentiated hOPC1 and hOPC2, the expression of OCT4, a representative marker of undifferentiated stem cells, was significantly decreased, while Pre-OPCs markers OLIG2 and NKX2.2 appeared and their expression increased significantly, and OPCs markers PDGFRa and SOX10 also gradually increased th...
Embodiment 3
[0103] Example 3 Therapeutic effect of human oligodendrocyte progenitor cells hOPC obtained by induction and culture in the present invention on ischemic stroke in rats
[0104] After the present invention uses the suture method to establish the middle cerebral artery occlusion-reperfusion (MCAO / R) model in rats, various neurobehavioral scores, TTC staining and other organizational methods are used to judge the infarct size, nerve injury site and degree, and then to To assess neurological injury in rats with ischemic stroke, determine the site of cell injection. Before the injection of hOPC, the immunosuppressant FK506 (1 mg / kg, intraperitoneal injection) was started to reduce or avoid the allogeneic immune rejection of the human cells by the rat body. The hOPCs were injected into the ischemic area and the surrounding white matter area of the stroke model. The injection method is to inject 250,000-400,000 cells into each point in the cerebral cortex on the same side as the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com