Artificial G-quadruplex DNA metalloenzyme and application thereof
A quadruplex and metalloenzyme technology, applied in the field of artificial G-quadruplex DNA metalloenzyme, can solve the problem of low ee%, and achieve the effects of simple operation, mild reaction conditions and good enantioselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
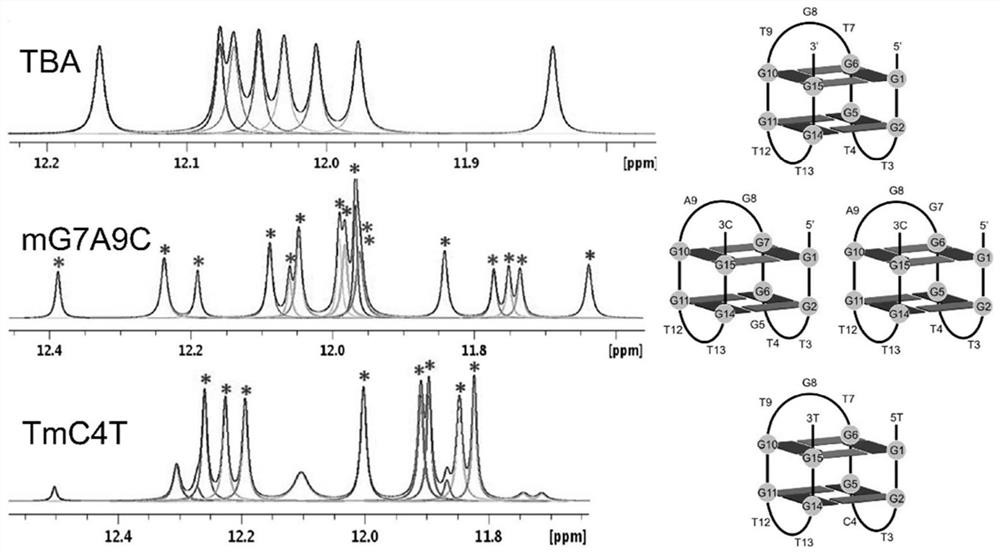
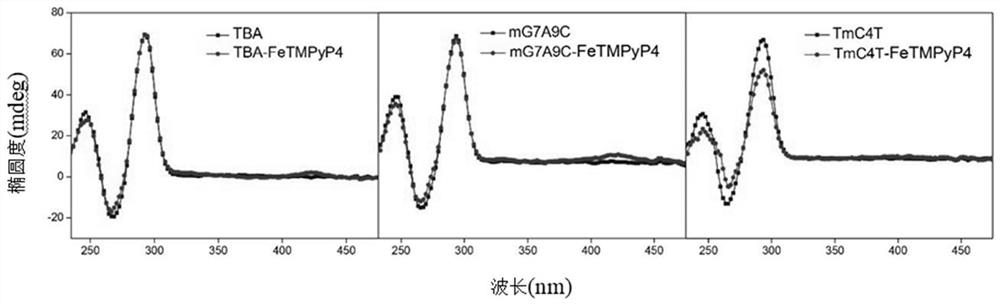
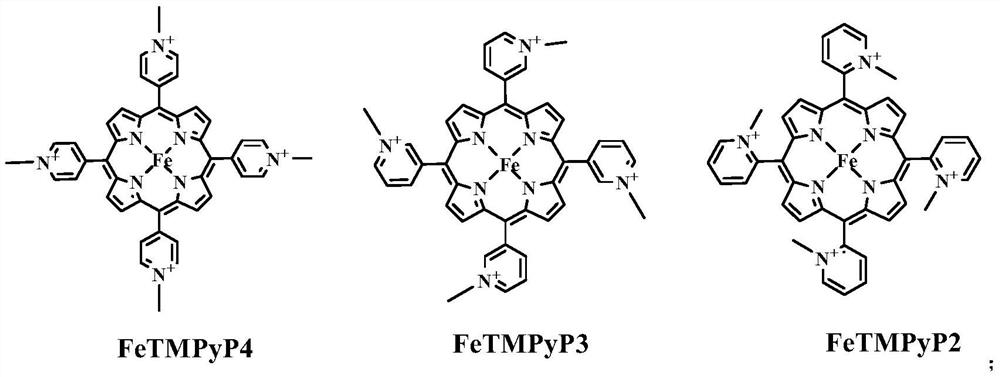
[0053] Add potassium phosphate (KPi) buffer solution (10mM, pH 7.0) to a 10mL Schlenk tube with a stirring bar, argon for 10min, add DNA (12.5μM): GGTTGGTGTGGTTGG (TBA), under the protection of argon (bubbling , open to the atmosphere), then add FeTMPyP4 (12.5μM), and stir for 10min to generate artificial G-quadruplex DNA metalloenzyme in situ (see figure 1 and figure 2 ), the ratio of artificial G-quadruplex DNA to FeTMPyP4 is 1:1. Sodium dithionite (5mM), styrene (30mM) and ethyl diazoacetate (10mM) were added to the above system, reacted and stirred for 2h at 4°C, and the reaction was terminated, extracted with ethyl acetate, and the product (1RS , 2RS)-2-phenylcyclopropane-1-carboxylic acid ethyl ester [(1RS,2RS)-Ethyl 2-phenylcyclopropane-1-carboxylate], the product is separated and detected by chiral high-performance liquid chromatography, and the chiral column uses OJH column. Analytical results: 48% enantioselectivity.
[0054] Product analysis
[0055] 1:10), t...
Embodiment 2
[0057] Add potassium phosphate (KPi) buffer solution (10mM, pH 7.0) to a 10mL Schlenk tube with a stirring bar, argon for 10min, add DNA (12.5μM): TGGTCGGTGTGGTTGGT (TmC4T), under argon protection (bubbling , open to the atmosphere), then add FeTMPyP4 (12.5μM), and stir for 10min to generate artificial G-quadruplex DNA metalloenzyme in situ (see figure 1 and figure 2 ), the ratio of artificial G-quadruplex DNA to FeTMPyP4 is 1:1. Add sodium dithionite (5mM), styrene (30mM) and ethyl diazoacetate (10mM) to the above system, react and stir for 2h at 4°C, then end the reaction, extract with ethyl acetate, and obtain the product (1RS , 2RS)-2-phenylcyclopropane-1-carboxylic acid ethyl ester [(1RS,2RS)-Ethyl 2-phenylcyclopropane-1-carboxylate], the product is separated and detected by chiral high-performance liquid chromatography, and the chiral column uses OJH column. Analytical results: -79% enantioselectivity.
[0058] Product analysis
[0059] 1:10), to afford the produ...
Embodiment 3
[0061] Add potassium phosphate (KPi) buffer solution (10mM, pH 7.0) to a 10mL Schlenk tube with a stirring bar, argon for 10min, add DNA (12.5μM): TGGTCGGTGTGGTTGGT (TmC4T), under argon protection (bubbling , open to the atmosphere), then add FeTMPyP4 (25μM), stir for 10min to generate artificial G quadruplex DNA metalloenzyme in situ, the ratio of artificial G quadruplex DNA and FeTMPyP4 is 1:2. Add sodium dithionite (5mM), styrene (30mM) and ethyl diazoacetate (10mM) to the above system, react and stir for 2h at 4°C, then end the reaction, extract with ethyl acetate, and obtain the product (1RS , 2RS)-2-phenylcyclopropane-1-carboxylic acid ethyl ester [(1RS,2RS)-Ethyl 2-phenylcyclopropane-1-carboxylate], the product is separated and detected by chiral high-performance liquid chromatography, and the chiral column uses OJH column. Analytical results: -73% enantioselectivity.
[0062] Product analysis
[0063] Purified by column chromatography (SiO 2 , EtOAc:pentane=1:10)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com