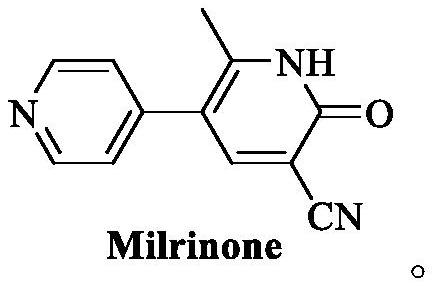
Synthesis method of milrinone
A synthetic method, the technology of milrinone, applied in the field of drug synthesis, can solve the problems of complex synthetic route, expensive price, difficult operation, etc., and achieve the effect of simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
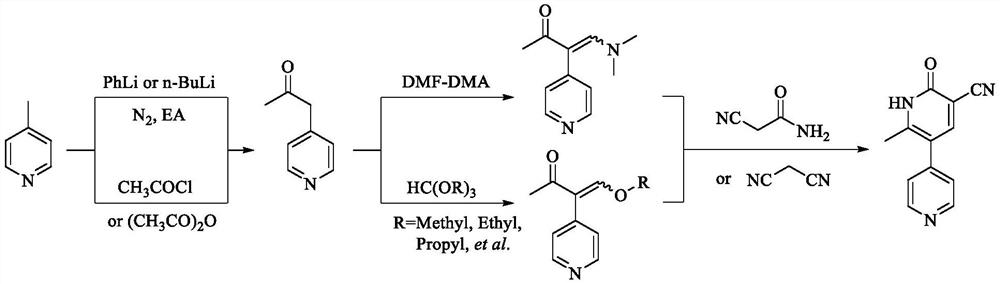
Method used
Image
Examples
Embodiment 1
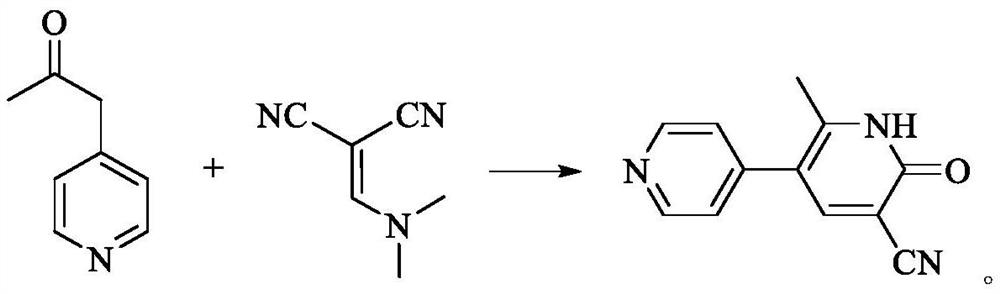
[0046]Add 1-(4-pyridyl)-2-propanone (135.05g, 1.0mol), α-(N,N-dimethylaminomethylene) malononitrile (145.23g, 1.2mol) into ethanol (1000ml) After stirring evenly, add 7.5mol / L sodium hydroxide solution to adjust the pH value to 13-14, and then control the temperature for reflux reaction. After the reaction is completed, the reaction solution is lowered to room temperature, and acetic acid is added to adjust the pH value of the solution to 6-7. crystallized, filtered, and the filter cake was washed with 40-50° C. purified water (500 ml) for hot beating, and dried to obtain off-white solid milrinone with a yield of 92.8% and a purity of 99.97%.
Embodiment 2
[0048] Add 1-(4-pyridyl)-2-propanone (135.03g, 1.0mol) α-(N,N-dimethylaminomethylene) malononitrile (121.06g, 1.0mol) into ethanol (700ml) , after stirring evenly, add 7.0mol / L potassium hydroxide solution to adjust the pH value to 13-14, control the temperature for reflux reaction, after the reaction is completed, the reaction solution is lowered to room temperature, add acetic acid to adjust the pH value of the solution to 6-7, stir and crystallize , filtered, and the filter cake was washed with 40-50° C. purified water (400 ml) for hot beating, and dried to obtain off-white solid milrinone with a yield of 90.4% and a purity of 99.94%.
Embodiment 3
[0050] Add 1-(4-pyridyl)-2-propanone (135.00g, 1.0mol), α-(N,N-dimethylaminomethylene)malononitrile (108.92g, 0.9mol) into ethanol (700ml) After stirring evenly, add 7.5mol / L sodium hydroxide solution to adjust the pH value to 13-14, and then control the temperature for reflux reaction. After the reaction is completed, the reaction solution is lowered to room temperature, and acetic acid is added to adjust the pH value of the solution to 6-7. crystallized, filtered, and the filter cake was washed with 40-50° C. purified water (400 ml) for hot beating, and dried to obtain off-white solid milrinone with a yield of 86.9% and a purity of 99.78%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com