Aldehyde group protection method for biomass-based furan compound
A compound and biomass technology, applied in chemical instruments and methods, physical/chemical process catalysts, organic chemistry, etc., can solve the problems of column chromatography time-consuming, deprotection, solvents, etc., achieve low prices, avoid time-consuming and raw materials, The effect of reducing industrial costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
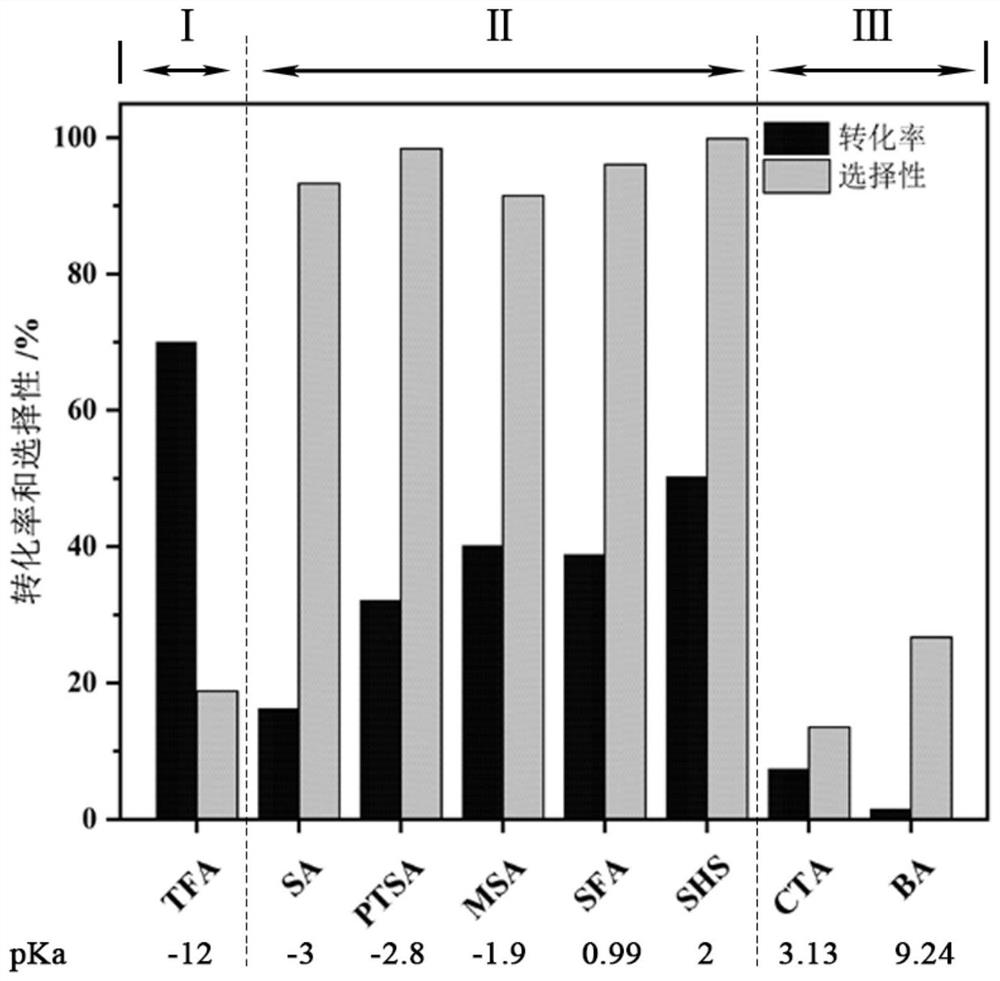
[0055] The present embodiment has carried out the screening of catalyst to the aldehyde protection method of the present invention, and specific process comprises:
[0056] Take 1mmol of trifluoromethanesulfonic acid (TFA), sulfuric acid (SA), p-toluenesulfonic acid (PTSA), methanesulfonic acid (MSA), sulfamic acid (SFA), anhydrous sodium bisulfate (SHS), anhydrous Citric acid (CTA) and boric acid (BA) were placed in 10mL clean reaction tubes, respectively marked as serial numbers 1-8, and then 1mmol furfural, 1mmol 1,3-propanediol and 3mL solvent dichloromethane were added, and the The reaction was carried out in an oil bath for 3 h, and after the reaction was completed, 1 mmol of toluene was added as an internal standard, and samples were taken for detection by gas chromatography. The catalytic effect is shown in Table 1 and figure 1 shown.
[0057] Table 1 utilizes Bronsted acid catalyst to prepare the catalytic effect of furfural acetal compound
[0058]
[0059] Thi...
Embodiment 2
[0063] This embodiment optimizes reaction temperature and reaction time, and concrete process is as follows:
[0064] The amount of furfural used in this experiment was 1 mmol, 4 mmol 1,3-propanediol, 1 mmol catalyst, 3 mL solvent methylene chloride, and 1 mmol internal standard toluene; the reaction was carried out under the conditions in Table 2, and samples were taken at different time points for gas chromatography detection. The specific experimental conditions and results are shown in Table 2:
[0065] The catalytic effect of sulfamic acid under the different reaction conditions of table 2
[0066]
[0067]
[0068] According to the contents of Table 2, it can be seen that with the increase of reaction temperature and reaction time, the conversion rate and yield of furfural show a trend of first increasing and then decreasing, wherein the optimum reaction temperature of this reaction is 60 °C, and the optimum reaction time is 180min.
Embodiment 3
[0070] This embodiment investigates the different molar ratios of furfural and 1,3-propanediol, using sulfamic acid as a catalyst to prepare furfural acetal products, the reaction reagents used in this experiment, the amount of each reagent used in the experiment in Example 2 The experiment of No. 17 is the same, except that the ratio of the reaction raw materials is changed. The specific experimental conditions and results are shown in Table 3:
[0071] The catalytic effect of sulfamic acid under the different raw material ratios of table 3
[0072]
[0073] It can be seen from Table 3 that with the change of the molar ratio of furfural and 1,3-propanediol, the conversion rate of furfural and the selectivity of acetal showed a trend of first increasing and then decreasing, among which the best furfural and 1,3-propanediol The molar ratio of propylene glycol is 1:4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com