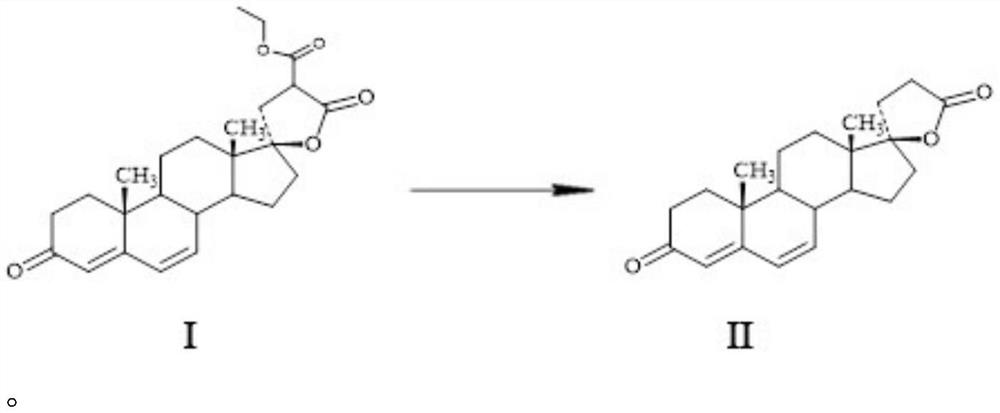
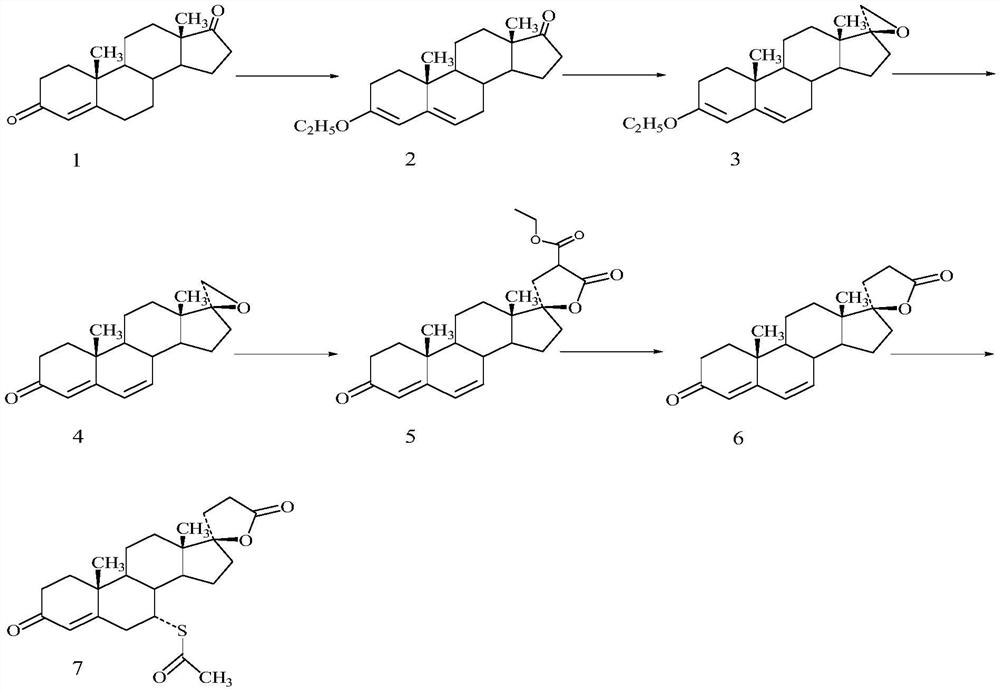
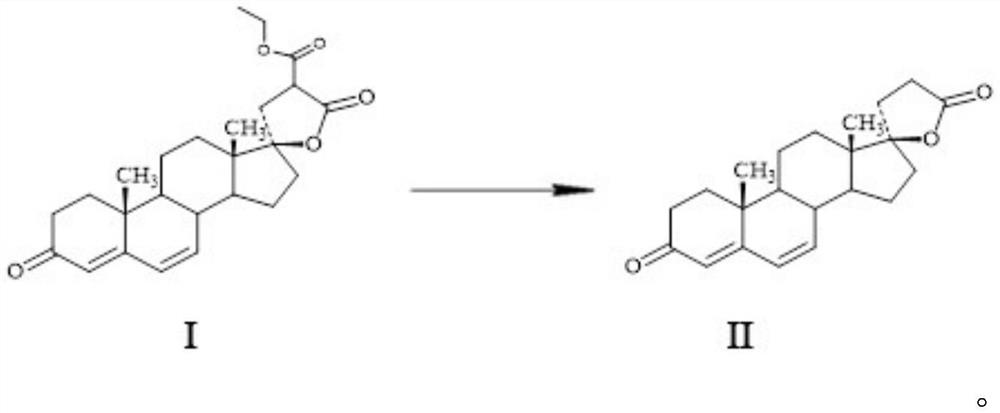
Method for preparing spirolactone intermediate canrenone
A technology of canrenone and intermediates, which is applied in the field of drug synthesis, can solve problems such as inconvenient operation, easy production of impurities, and impact on the quality of canrenone, and achieve the effects of reducing raw material costs, easy recycling, and reducing thermally degraded impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] A method for preparing spironolactone intermediate canrenone, comprising the following steps: dissolving 25g of lactone I in 500ml of toluene, adding 50ml of DMAC and 10g of poly-4-vinylpyridine, stirring and reacting at 80°C, and after 6 hours Add 300ml of water, stir and separate the water layer, filter the organic phase and concentrate under reduced pressure (absolute pressure less than 0.02MP, temperature less than 80°C) to a small volume (the amount of solvent is about 60-75ml), cool down and keep warm at 0-10°C After 2 hours, 17.8 g of solid were obtained after filtration. After testing: the melting point of the obtained product is 148-150°C (literature value 149-151°C), the molar yield is 80.40%, the product is determined to be canrenone by NMR detection, and its purity is 98.6% by HPLC detection.
Embodiment 2
[0022] A method for preparing spironolactone intermediate canrenone, comprising the following steps: dissolving 25 grams of lactone I in 250 ml of cyclohexane, adding 20 ml of NMP and 12.5 g of poly-4-vinylpyridine, and stirring at 50 ° C for reaction After 6 hours, add 200ml of water, separate the water layer after stirring, and concentrate under reduced pressure after filtering the organic phase (absolute pressure is less than 0.02MP, temperature is less than 50°C) to a small volume (solvent amount is about 60-75ml), cooling, 8 It was kept at -10°C for 2 hours, and 17.4 g of solid was obtained by filtration. After testing: the melting point of the obtained product is 150-151° C. (literature value 149-151° C.), the molar yield is 78.59%, the product is determined to be canrenone by NMR detection, and its purity is 98.8% by HPLC detection.
Embodiment 3
[0024] A method for preparing spironolactone intermediate canrenone, comprising the following steps: dissolving 20 grams of lactone I in 100 ml of methyl tetrahydrofuran, adding 4 ml of DMF and 1 g of poly-4-vinylpyridine, stirring at 65 ° C, Add 100ml of water after 6 hours, separate the water layer after stirring, filter the organic phase and concentrate under reduced pressure (absolute pressure less than 0.02MP, temperature less than 60°C) to a small volume (solvent amount is about 50-60ml), cool down, 0- It was kept at 10°C for 2 hours, and 15.2 g of solid was obtained by filtration. After testing: the melting point of the obtained product is 150-151° C. (literature value 149-151° C.), the molar yield is 85.82%, the product is determined to be canrenone by NMR detection, and its purity is 98.7% by HPLC detection.
[0025] The part characteristic hydrogen data of embodiment 1-3 gained product is as follows:
[0026]
[0027] 1 H NMR (400MHz, CDCl 3 )δ6.16-6.08(m,2H),5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com