Preparation method of formamide compound
A technology for amine compounds and formamides is applied in the field of preparation of formamide compounds, and achieves the effects of good catalyst repeatability, simple operation and post-processing, and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the synthesis of N-methylformanilide
[0036] In a 20mL autoclave, add nanoporous palladium catalyst PdNPore-1 (2.7mg, 0.025mmol), N-methylaniline (53.5mg, 0.5mmol), phenylsilane (216.4mg, 2mmol), acetonitrile (2mL ), deionized water (10 μL) and carbon dioxide (1.0 MPa), placed on a magnetic stirrer at 60 ° C for 20 h, column chromatography (silica gel, 200-300 mesh; developer, petroleum ether, ethyl acetate = 2: 1) Obtain 53.7 mg of N-methylformanilide, yield 85%
[0037]
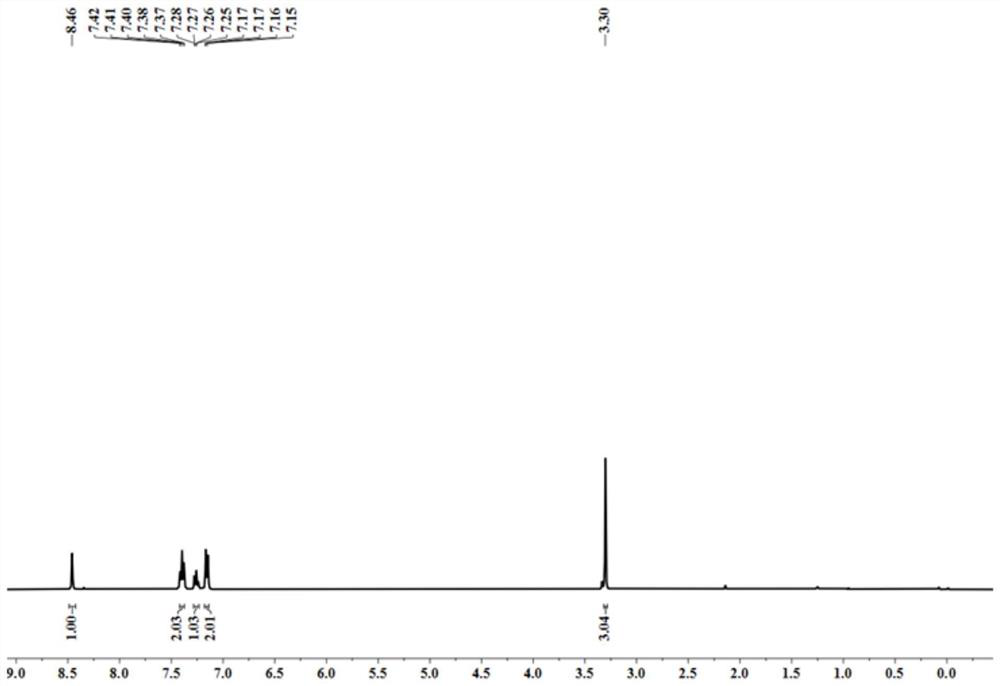
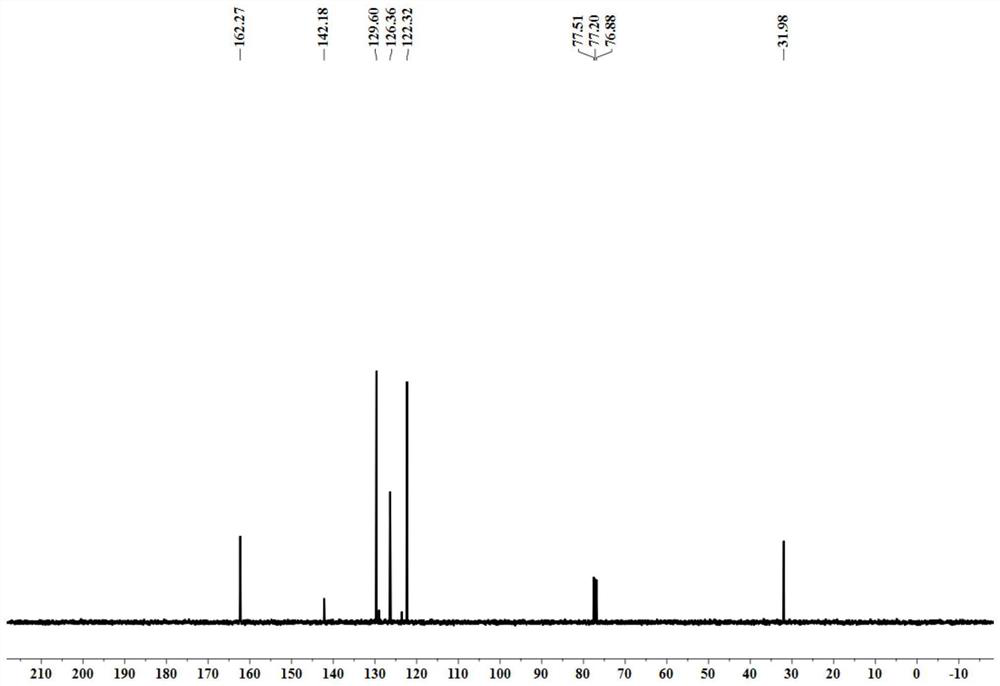
[0038] yellow oily liquid; 1 H NMR (CDCl 3 ,400MHz)δ8.46(s,1H),7.42-7.37(m,2H),7.28-7.24(m,1H),7.16(d,J=8.0Hz,2H),3.30(s,3H); 13 C NMR (CDCl 3 ,100MHz) δ162.3, 142.2, 129.6, 126.4, 122.3, 32.0.
Embodiment 2
[0039] Embodiment 2: the synthesis of N-methyl-4-methoxyformanilide
[0040] In a 20mL autoclave, add nanoporous palladium catalyst PdNPore-1 (2.7mg, 0.025mmol), N-methyl-4-methoxyaniline (68.5mg, 0.5mmol), phenylsilane (216.4mg, 2mmol), acetonitrile (2mL), deionized water (10μL) and carbon dioxide (2.0MPa), placed on a magnetic stirrer at 70 ° C for 24h, column chromatography (silica gel, 200-300 mesh; developer, petroleum ether, Ethyl acetate = 2:1) to obtain 75.1 mg of N-methyl-4-methoxyformanilide with a yield of 91%.
[0041]
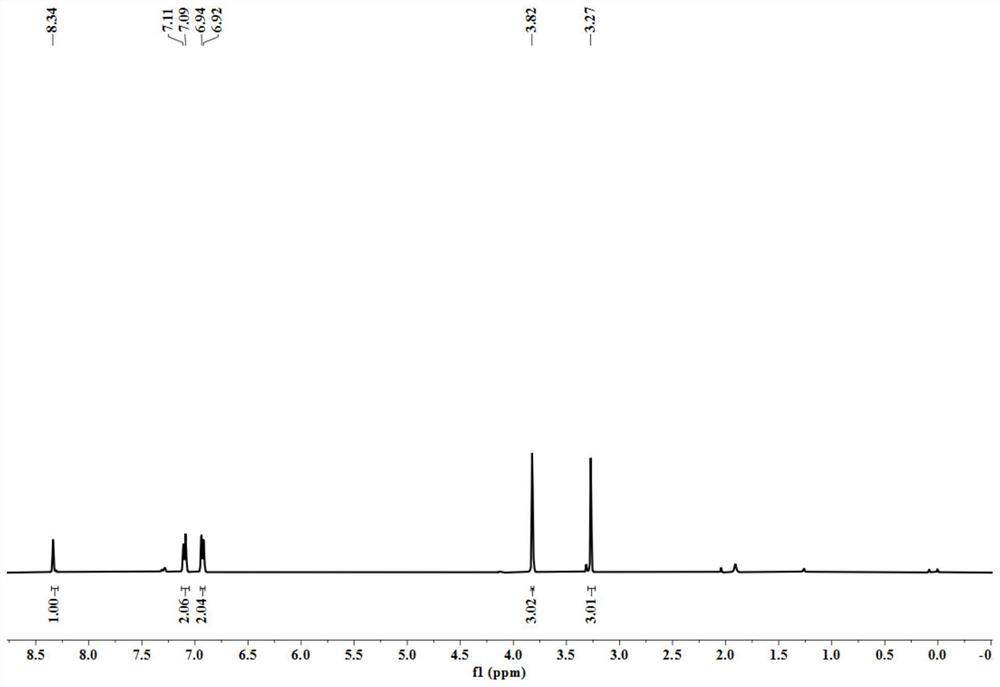
[0042] yellow oily liquid; 1 H NMR (CDCl 3 ,400MHz)δ8.34(s,1H),7.10(d,J=8.0Hz,2H),6.93(d,J=8.0Hz,2H),3.83(s,3H),3.27(s,3H); 13 C NMR (CDCl 3,100MHz)δ162.5,158.3,135.3,124.7,114.8,55.5,32.7.
Embodiment 3
[0043] Embodiment 3: the synthesis of N-allyl formanilide
[0044] In a 20mL autoclave, add nanoporous palladium catalyst PdNPore-1 (2.7mg, 0.025mmol), N-allylaniline (66.5mg, 0.5mmol), phenylsilane (270.5mg, 2.5mmol), acetonitrile (2mL), deionized water (10μL) and carbon dioxide (1.0MPa), placed on a magnetic stirrer at 70 ° C for 24h, column chromatography (silica gel, 200-300 mesh; developer, petroleum ether, ethyl acetate = 2:1) to obtain 64.5mg of N-allylformanilide, yield 80%
[0045]
[0046] Yellow oily liquid. 1 H NMR (CDCl 3 ,400MHz)δ8.40(s,1H),7.32-7.27(m,2H),7.20-7.17(m,1H),7.10(d,J=8.0Hz,2H),5.81-5.71(m,1H) ,5.12-5.07(m,2H),4.33(d,J=8.0Hz,2H); 13 C NMR (CDCl 3 ,100MHz)δ162.0,141.2,132.5,130.0,126.7,123.5,117.6,47.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com