Preparation method of baloxavir intermediate
An intermediate, dimethoxyethane technology, applied in the field of preparation of baloxavir intermediates, can solve the problems of low conversion rate of compound E, difficult crystallization, low purity of compound D, etc., and achieve high product purity, Simple operation and mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
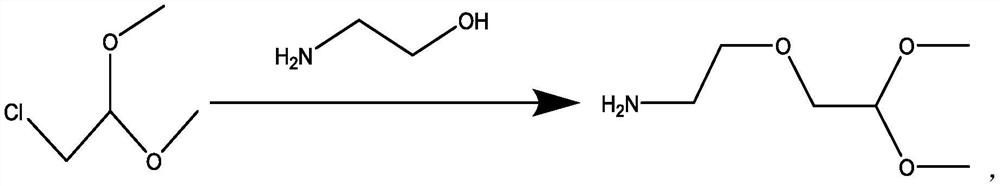
[0032] Add 247.6g of ethanolamine and 272.7g of sodium tert-butoxide into a 2L three-necked flask, stir, raise the temperature to 80-90°C, stir for 3h, add 101.0g of 2-chloroacetaldehyde dimethyl acetal dropwise, react at 90°C for 3h, and control in GC The detection reaction is complete.
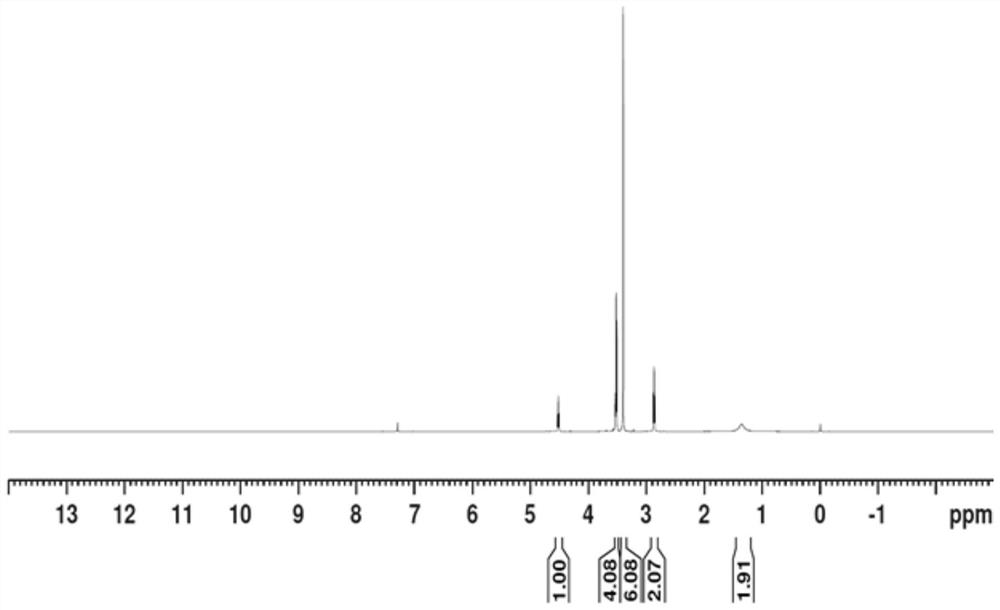
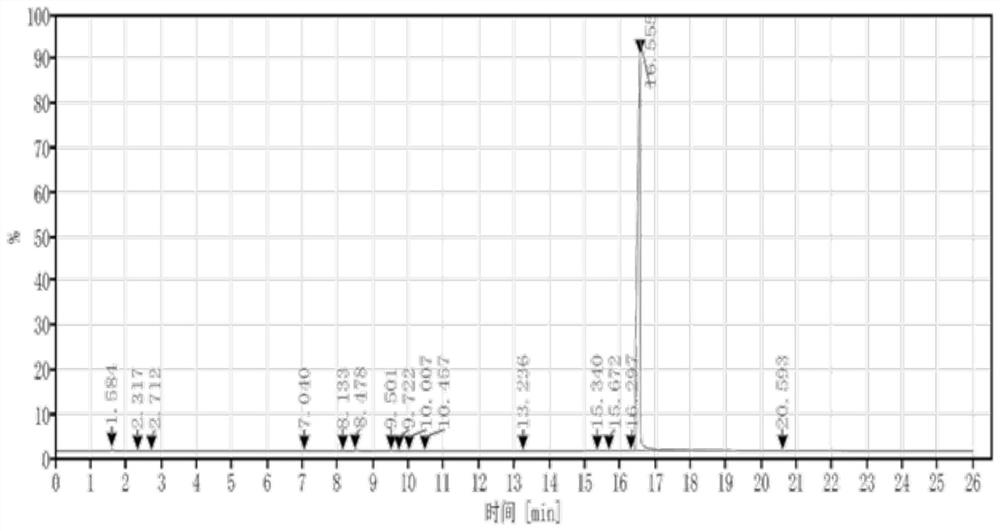
[0033] Post-treatment: add 12L of ice water, then add 12L of dichloromethane, stir for 30min, let stand to separate the liquid, wash the organic phase with 500ml of sodium sulfate solution, separate the liquid, concentrate the organic phase, and rectify with an oil pump to obtain 63.3g of the product, namely ( 2,-(2-Aminoethoxy)-1,1-dimethoxyethane, yield 52%, hydrogen spectrum, GC spectrum as Figure 1-2 shown.
Embodiment 2
[0035] Add 29.5g of ethanolamine and 31g of sodium tert-butoxide into a 500mL three-necked flask, stir, raise the temperature to 80-90°C, stir for 3h, add 10g of 2-chloroacetaldehyde dimethyl acetal dropwise, react at 90°C for 3h, and detect the reaction in GC End.
[0036] Post-processing: add 1L of ice water, then add 1L of dichloromethane, stir for 30min, let stand to separate the liquid, wash the organic phase with 50ml of sodium sulfate solution, separate the liquid, concentrate the organic phase, and rectify with an oil pump to obtain 6.6g of the product, namely ( 2,-(2-aminoethoxy)-1,1-dimethoxyethane, yield 55%.
Embodiment 3
[0038] Add 3.43g of ethanolamine and 3.47g of sodium tert-butoxide into a 100mL three-necked flask, stir, raise the temperature to 80-90°C, stir for 3h, add 1g of 2-chloroacetaldehyde dimethyl acetal dropwise, react at 90°C for 3h, and detect in GC The reaction is over.
[0039] Post-processing: add 100mL of ice water, then add 100mL of dichloromethane, stir for 30min, let stand to separate the liquid, wash the organic phase with 50ml of sodium sulfate solution, separate the liquid, concentrate the organic phase, and rectify with an oil pump to obtain 0.6g of the product, namely ( 2,-(2-aminoethoxy)-1,1-dimethoxyethane, yield 50%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com