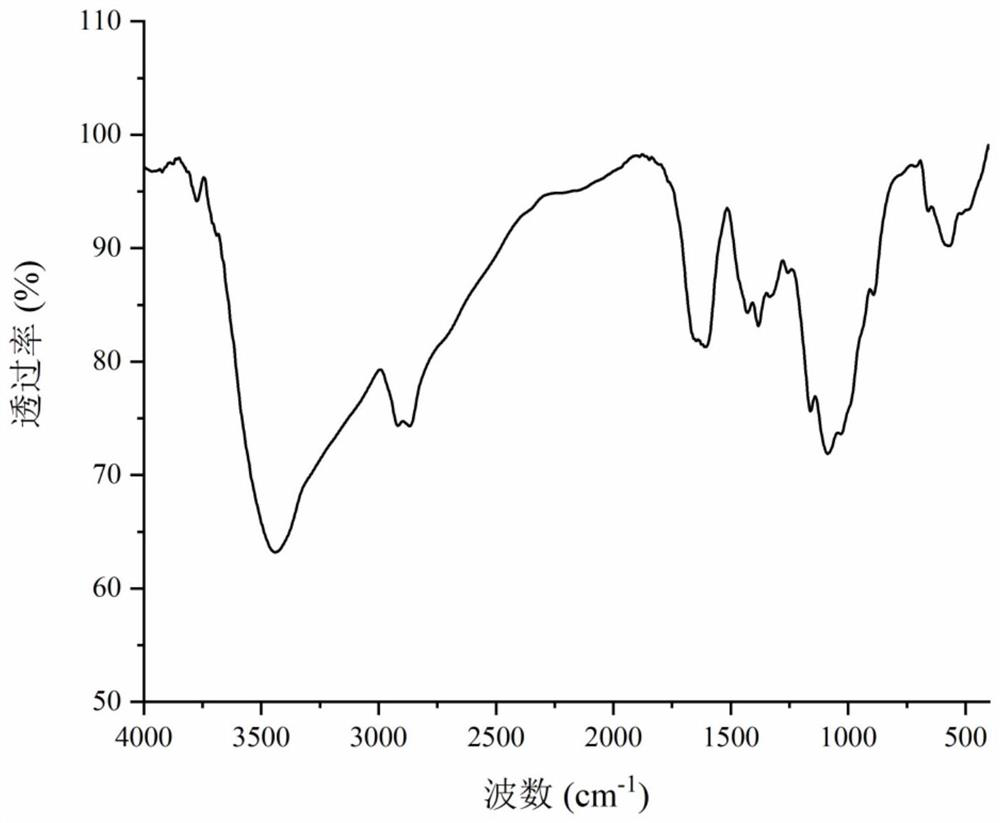
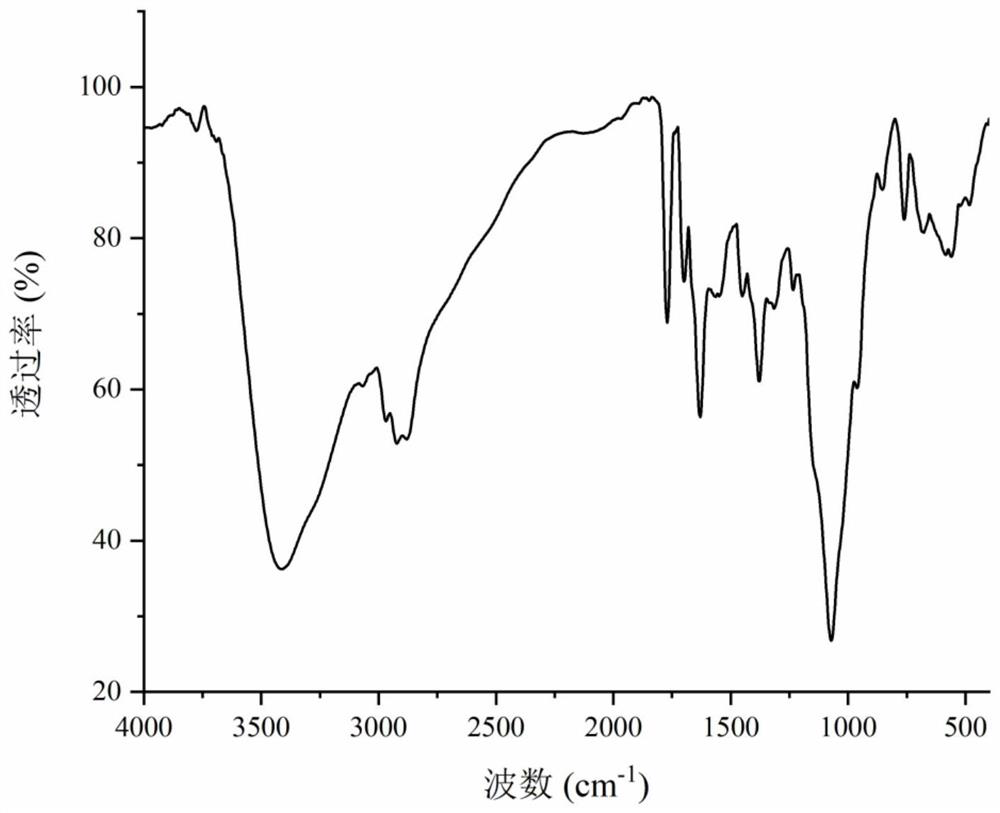
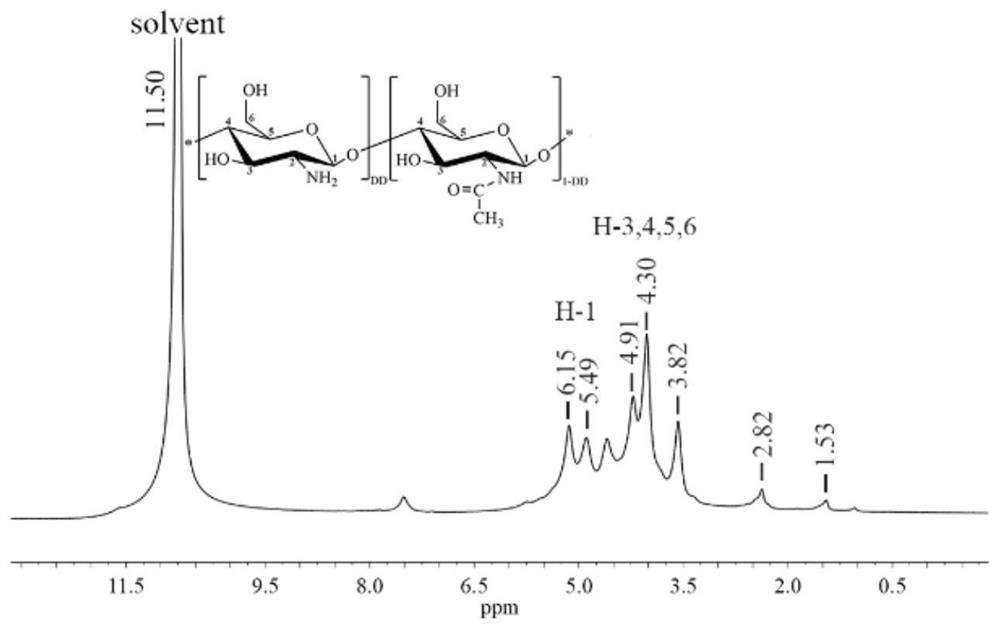
A kind of preparation method and use of cinnamic acid-modified hydroxypropyl chitosan derivative
A technology of hydroxypropyl chitosan and cinnamic acid, which is applied in the fields of botanical equipment and methods, chemicals for biological control, applications, etc., can solve the problems of not very common application, limited application value, low antibacterial activity, etc. , to achieve the effect of wide dissolution range, high substitution degree and yield, and improved water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] The preparation method of cinnamic acid-modified hydroxypropyl chitosan derivative comprises the following steps:
[0042] 1. Preparation of Hydroxypropyl Chitosan
[0043] The chitosan with a molecular weight of 100kDa and a degree of deacetylation of 80% was selected.
[0044]Add 15 mL of 50% sodium hydroxide aqueous solution to 5 g of chitosan, stir at room temperature for 3 hours to make it evenly mixed, and place it in a -20 °C refrigerator for 24 hours to fully alkalize and expand the chitosan; thaw and transfer the mixture Put it into a three-necked flask containing 50 mL of isopropanol, stir vigorously for 30 min at room temperature, add 1 mL of 25% tetramethylammonium hydroxide and 50 mL of propylene oxide under stirring, continue to stir at room temperature for 1 hour, and reflux at 45°C for 6 hours, the reaction is complete Then, it was cooled to room temperature, purified by ultrapure water dialysis for 72h (the cut-off molecular weight of the dialysis bag ...
Embodiment 2
[0055] The preparation method of cinnamic acid-modified hydroxypropyl chitosan derivative comprises the following steps:
[0056] 1. Preparation of Hydroxypropyl Chitosan
[0057] The chitosan with a molecular weight of 100kDa and a degree of deacetylation of 80% was selected.
[0058] Add 15 mL of 50% sodium hydroxide aqueous solution to 5 g of chitosan, stir at room temperature for 3 hours to make it evenly mixed, and place it in a -20 °C refrigerator for 24 hours to fully alkalize and expand the chitosan; thaw and transfer the mixture Put it into a three-necked flask containing 50 mL of isopropanol, stir vigorously for 30 min at room temperature, add 1 mL of 25% tetramethylammonium hydroxide and 50 mL of propylene oxide under stirring, continue to stir at room temperature for 1 hour, and reflux at 45°C for 6 hours, the reaction is complete Then, it was cooled to room temperature, purified by ultrapure water dialysis for 72h (the cut-off molecular weight of the dialysis bag...
Embodiment 3
[0063] The preparation method of cinnamic acid-modified hydroxypropyl chitosan derivative comprises the following steps:
[0064] 1. Preparation of Hydroxypropyl Chitosan
[0065] The chitosan with a molecular weight of 100kDa and a degree of deacetylation of 80% was selected.
[0066] Add 15 mL of 50% sodium hydroxide aqueous solution to 5 g of chitosan, stir at room temperature for 3 hours to make it evenly mixed, and place it in a -20 °C refrigerator for 24 hours to fully alkalize and expand the chitosan; thaw and transfer the mixture Put it into a three-necked flask containing 50 mL of isopropanol, stir vigorously for 30 min at room temperature, add 1 mL of 25% tetramethylammonium hydroxide and 50 mL of propylene oxide under stirring, continue to stir at room temperature for 1 hour, and reflux at 45°C for 6 hours, the reaction is complete Then, it was cooled to room temperature, purified by ultrapure water dialysis for 72h (the cut-off molecular weight of the dialysis bag...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| degree of deacetylation | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com