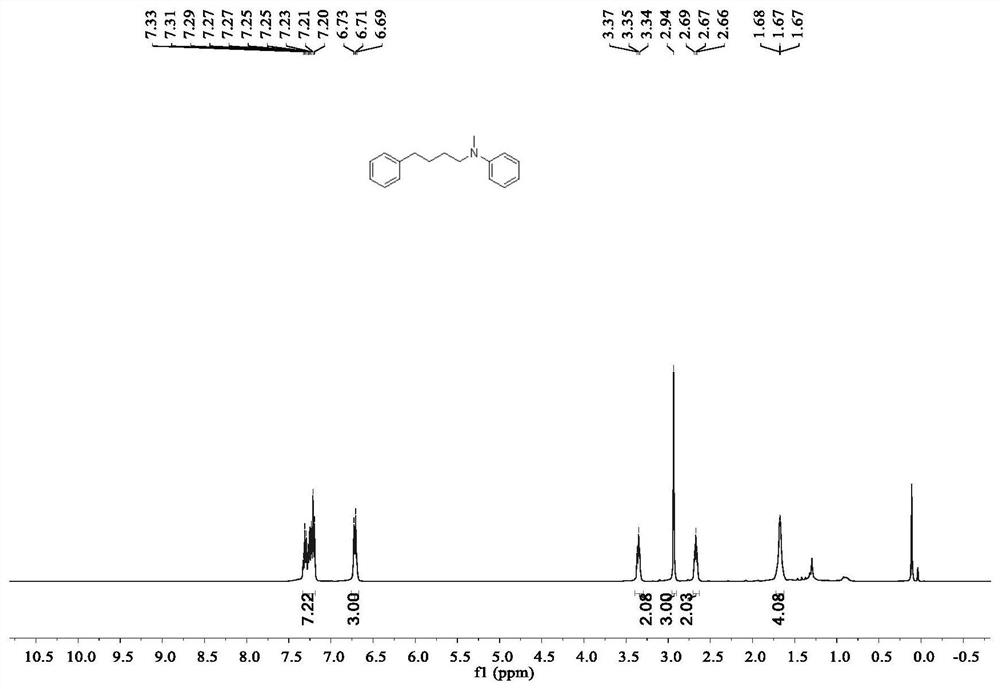
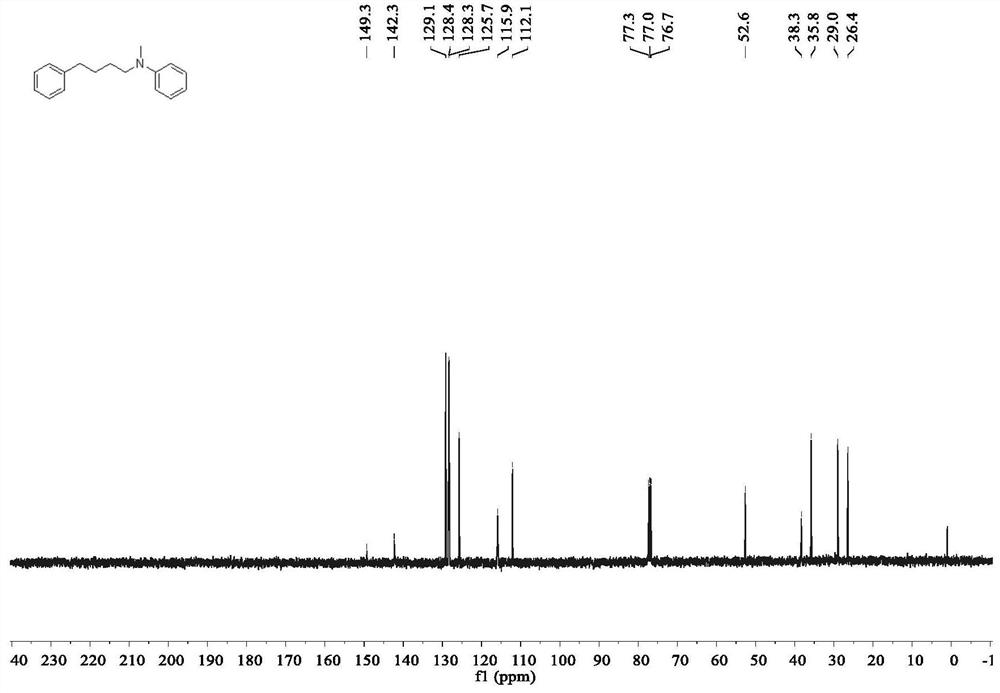
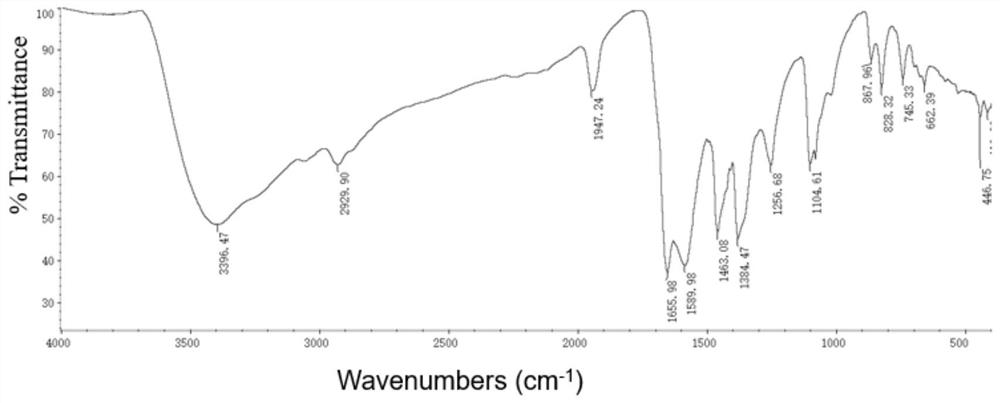
Method for preparing linear amine through selective hydroamine methylation of olefin
A technology of hydroamine methyl and linear amine, which is applied in the direction of chemical instruments and methods, condensation/addition reactions to prepare amino compounds, organic compounds/hydrides/coordination complex catalysts, etc., which can solve the difficulty of separation and purification and operation Cost, increase the difficulty and cost of separation and purification, and decrease the yield of amine products, etc., to achieve good industrial application value, high efficiency and selectivity, and avoid post-processing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1, the preparation of azacyclic carbene metal coordination polymer 1
[0046]
[0047] The synthetic method of the azacyclic carbene rhodium coordination polymer designed by the present invention, its process is as follows:
[0048] Add bis-imidazolium methyl salt ligand (1 mmol) and metal precursor [Rh(COD)Cl] to a 100 mL bottle under nitrogen 2 (1.0mmol), DMF (15mL), stir well at room temperature. Subsequently, 2 mL of LiHMDS (1M solution in THF) was added dropwise to the system, sealed and placed in an oil bath at 80° C. for 24 h of reaction. After the reaction, the system was cooled to room temperature, transferred to a 10mL centrifuge tube for centrifugation, and washed with DMF, deionized water, and methanol in sequence until the filtrate was clear and transparent, and the solid obtained by centrifugation was subjected to Soxhlet extraction with methanol, and vacuum-dried , the corresponding coordination polymer can be obtained. Dark brown solid, ...
Embodiment 2
[0049] Embodiment 2, the preparation of azacyclic carbene metal coordination polymer 2a:
[0050]
[0051] Add bis-imidazolium methyl salt ligand (1 mmol) and metal precursor [Rh(COD)Cl] to a 100 mL bottle under nitrogen 2 (1.0mmol), DMF (15mL), stir well at room temperature. Subsequently, 2 mL of LiHMDS (1M solution in THF) was added dropwise to the system, sealed and placed in an oil bath at 80° C. for 24 h of reaction. After the reaction, the system was cooled to room temperature, transferred to a 10mL centrifuge tube for centrifugation, and washed with DMF, deionized water, and methanol in sequence until the filtrate was clear and transparent, and the solid obtained by centrifugation was extracted by Soxhlet with methanol, and dried in vacuo , the corresponding coordination polymer can be obtained. Dark brown solid, yield 97%; IR(KBr pellet)ν436.72,727.09,744.28,816.01,861.10,923.91,948.90,1058.91,1247.16,1372.27,1471.17,1612.26,1659.753,1950.0726cm -1 .Elementalanal...
Embodiment 3
[0052] Embodiment 3, the preparation of azacyclic carbene metal coordination polymer 2b:
[0053]
[0054] Add bis-imidazolium methyl salt ligand (1 mmol) and metal precursor [Rh(COD)Cl] to a 100 mL bottle under nitrogen 2 (1.0mmol), DMF (15mL), stir well at room temperature. Subsequently, 2 mL of LiHMDS (1M solution in THF) was added dropwise to the system, sealed and placed in an oil bath at 80° C. for 24 h of reaction. After the reaction, the system was cooled to room temperature, transferred to a 10mL centrifuge tube for centrifugation, and washed with DMF, deionized water, and methanol in sequence until the filtrate was clear and transparent, and the solid obtained by centrifugation was extracted by Soxhlet with methanol, and dried in vacuo , the corresponding coordination polymer can be obtained. Dark brown solid, yield 93%; IR(KBr pellet)ν446.63,726.43,747.38,817.65,862.32,924.07,1087.50,1246.70,1372.80,1470.27,1612.15,1663.47,1950.29,2953.95,4cm -1 .Elemental ana...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com