Relugolix intermediate and preparation method thereof
A volume and time technology, which is applied in the field of relugoli intermediates and their preparation, can solve the problems of unsuitability for industrial production, excessive heavy metal elements, and low product purity, and achieves heavy metal residues up to standard, high total yield, and high purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
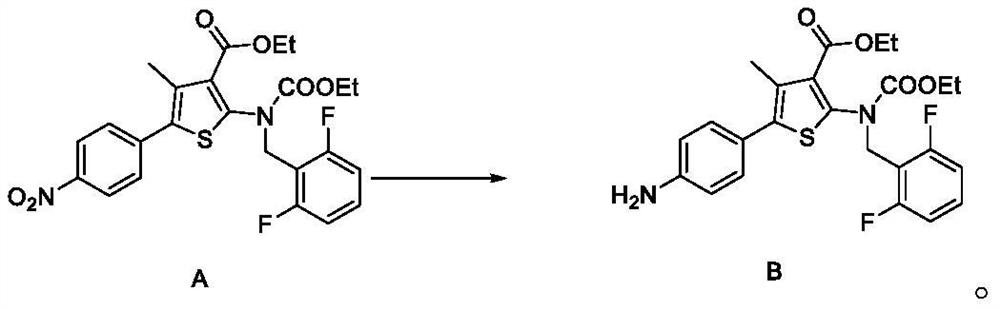
[0148] The synthesis of embodiment 1 compound B
[0149]
[0150] Compound A (80.0g) is added methanol (640mL) in a nitrogen atmosphere, 10%Pd-C (8.0g, described percentage refers to the percentage that the quality of palladium accounts for the total mass of palladium carbon) is added thereto, and the The mixture was stirred under hydrogen balloon pressure at 25±5°C for 4-6 hours. Activated carbon and catalyst were filtered off and washed with methanol (160 mL). Concentrate under reduced pressure at 45±5° C. until the weight is constant to obtain compound B as a yellow-brown oil with a yield of 99.0% and a purity of 98.0% by HPLC.
Embodiment 2
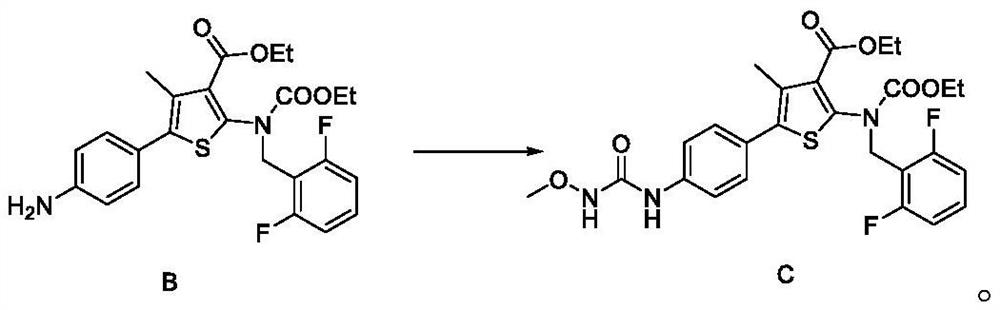
[0151] The synthesis of embodiment 2 compound C
[0152]
[0153]Acetonitrile (30 mL) and 1,1'-carbonyldiimidazole or (CDI, 5.01 g, 1.7 eq) were added to the reactor, and the mixture was stirred. Under stirring, triethylamine (1.56 g, 0.85 eq) was added thereto, and cooled to an internal temperature of 10±5°C. Methoxylamine hydrochloride (2.90 g, 1.91 eq) was added thereto in portions with stirring at a lower internal temperature of 30°C, and the container used for the reagent was washed with acetonitrile (5 mL). The mixture was stirred at an internal temperature of 25±5° C., and after it was confirmed that the mixture was dissolved, the solution was further stirred for 10 minutes or more. Then, Compound B (10.00 g) was added thereto under stirring, and the container used for the reagent was washed with acetonitrile (5 mL). The reaction mixture was heated to an internal temperature of 50±5°C, and stirred at the same temperature for 2-3 hours, cooled to an internal tempera...
Embodiment 3
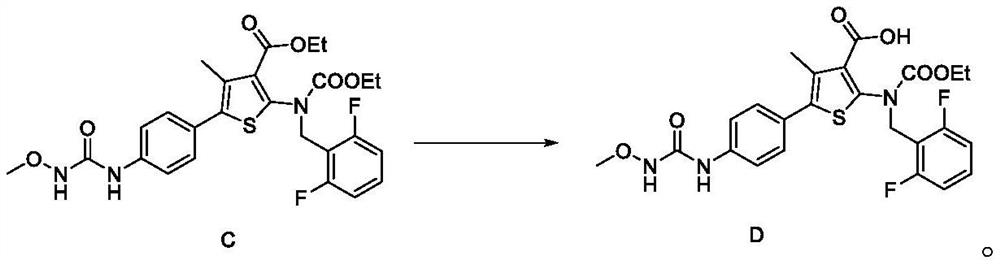
[0154] The synthesis of embodiment 3 compound D
[0155]
[0156] Compound C (10.0 g) was dissolved in ethanol (100 mL), 2N NaOH (45.7 ml, 5.0 eq) was added thereto, and stirred at an external temperature of 60° C. for about 6 hours. Concentrate the reaction solution until ethanol is basically removed, cool down to 20±5°C, and slowly adjust the pH to about neutral with 1N HCl (5.0eq). And stirred at room temperature for more than 2 hours, filtered to obtain a yellow solid, rinsed with water (20ml). The wet product was baked in an air blast oven at 50°C for 2 hours, then heated up to 60°C and baked overnight to obtain 7.7 g of off-white solid, compound D, with a yield of 81%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com