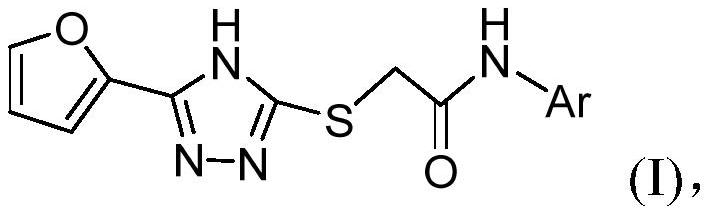
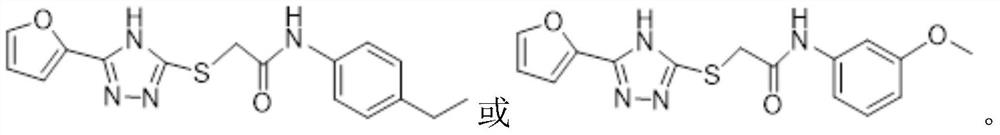
Triazole neuraminidase inhibitor as well as preparation method and application thereof
A technology of neuraminidase and inhibitors, applied in antiviral agents, organic chemistry, etc., can solve the problems of expensive raw materials and complex synthesis process of Tamiflu, achieve good neuraminidase inhibitory effect, simple synthesis method, Effect of excellent neuraminidase inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
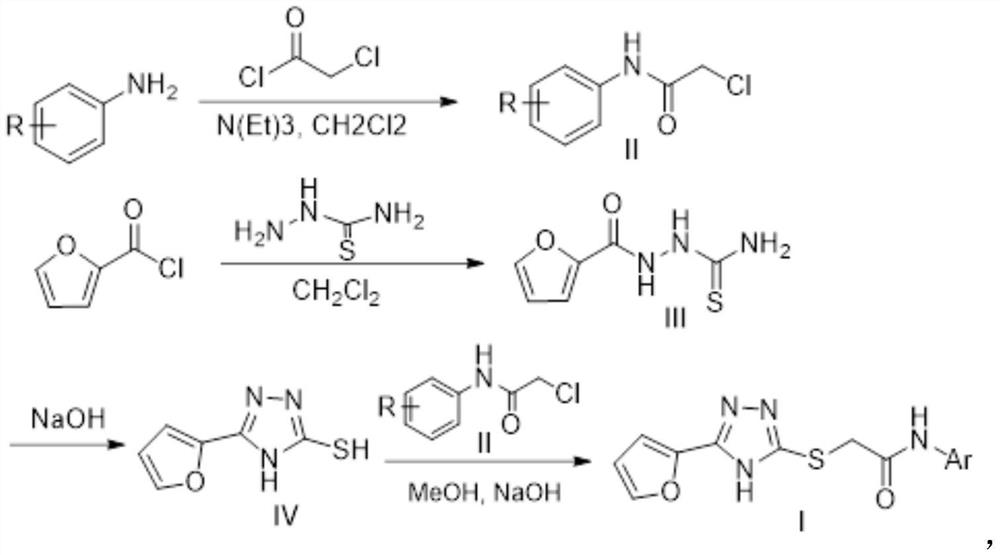
[0042] Described preparation method specifically comprises the following steps:
[0043] (1) Form a reaction system with substituted aniline and chloroacetyl chloride, and obtain the intermediate of formula (II) through post-treatment after the reaction;
[0044] (2) dissolving furoyl chloride and thiosemicarbazide in an organic solvent to form a reaction system, after the reaction, an intermediate of formula (Ⅲ) is obtained through post-processing;
[0045] (3) The intermediate of formula (Ⅲ) obtained in step (2) is dissolved in sodium hydroxide solution to form a reaction system, and the reaction is processed to obtain the intermediate of formula (Ⅳ);
[0046] (4) get the formula (IV) intermediate that step (3) obtains and the formula (II) intermediate that step (1) obtains and sodium hydroxide solution are dissolved in the organic solvent, react and obtain formula (I) through aftertreatment indicated inhibitors.
[0047] In step (1), triethylamine is used as the acid-bind...
Embodiment 1
[0054] A preparation method of triazole neuraminidase inhibitor, its structural formula is as shown in formula I:
[0055]
[0056] Concrete synthetic steps are as follows:
[0057] (1) Accurately weigh 0.61g (5mmol) of 3-ethylaniline and 0.51g (5mmol) of triethylamine in a 50mL round bottom flask, add 10mL of dichloromethane, use nitrogen protection, add 0.56g (5mmol) of chloroacetyl chloride ), placed in an ice-water bath at 0°C and stirred for 4 hours, then added 20 mL of dichloromethane to dilute the reaction, washed successively with 1M hydrochloric acid, saturated sodium bicarbonate solution and brine, and removed the dichloromethane by rotary evaporation to obtain the intermediate of formula (II) body.
[0058] (2) Accurately weigh 0.65g (5mmol) of 2-furoyl chloride and 0.46g (5mmol) of thiosemicarbazide into a 50mL round bottom flask, add 20mL of dichloromethane, place in an ice-water bath at 0°C and stir for 16 hours. Upon completion, the dichloromethane is remov...
Embodiment 2
[0090] A method for preparing a triazole neuraminidase inhibitor, its structural formula is as follows, and it is prepared by a method similar to Example 1.
[0091] N-(3,4-Dimethoxyphenyl)-2-((5-(furan-2-yl)-4H-1,2,4-triazol-3-yl)thio)acetamide
[0092]
[0093] White solid, 67% yield, IC 50 The value was 1.43 μM. 1 H NMR(500MHz,DMSO)δ14.46(s,1H),10.19(s,1H),7.87(s,1H),7.28(s,1H),7.07(d,J=10.0Hz,1H),6.99 (s, 1H), 6.88 (d, J=5.0Hz, 1H), 6.67 (s, 1H), 4.08 (s, 2H), 3.71 (s, 6H). 13C NMR (125MHz, DMSO) δ 165.67, 148.64, 145.09, 144.54, 143.81, 132.52, 112.14, 112.06, 111.16, 110.32, 104.36, 55.77, 55.39, 36.53. HRMS(ESI):361.0966[M-H]+
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com