Method for detecting related substances in pazufloxacin mesylate bulk drug by adopting HPLC
A technology of pazufloxacin mesylate and related substances, which is applied in the field of quality control of pazufloxacin mesylate, can solve problems such as unsatisfactory separation and ineffective detection of related substances, and achieve a suitable retention time Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
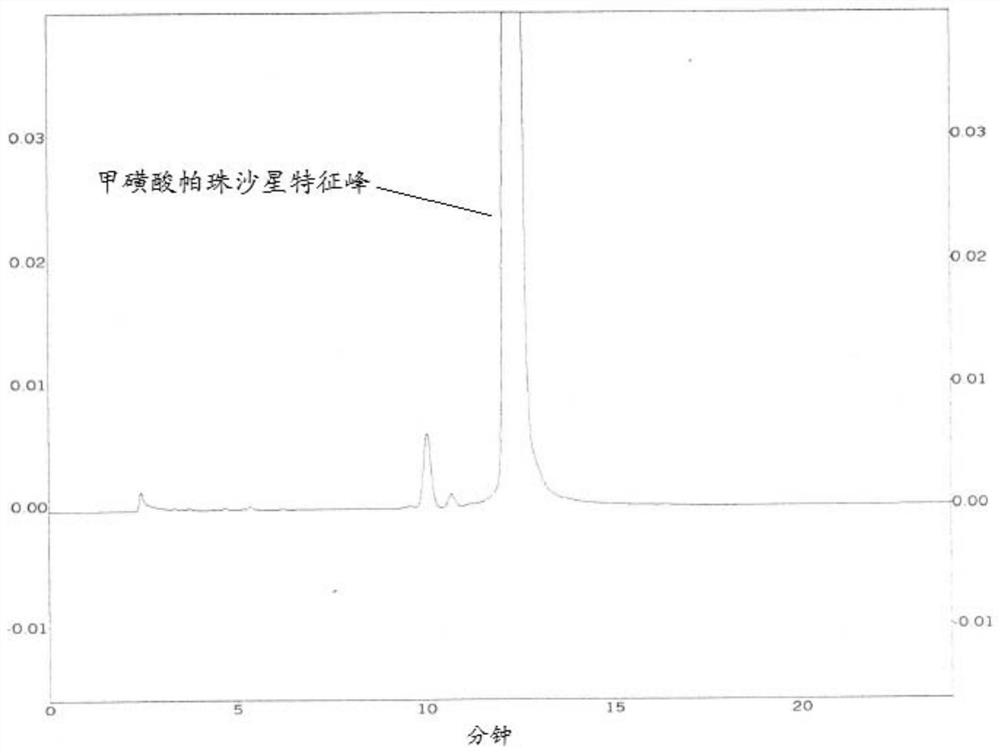
[0040] Embodiment 1 uses the first mobile phase Pazufloxacin mesylate crude drug to detect
[0041] The chromatographic conditions are as follows:
[0042] Stationary phase: Octadecylsilane bonded silica gel,
[0043] The first mobile phase: acetonitrile-10% triethylamine methanesulfonate-1M dipotassium hydrogen phosphate-water (30:10:7:170)
[0044] Flow rate: 1.0ml / min,
[0045] Detection wavelength: 254nm,
[0046] Injection volume: 20 μl.
[0047] The detection steps are as follows:
[0048] (1) Take 30mg of pazufloxacin mesylate crude drug, accurately weighed, put in a 100ml measuring bottle, add the first mobile phase to dissolve and dilute to the mark, shake well, and use it as the test solution.
[0049] (2) Under the condition of the first mobile phase, get need testing solution 20 μ l and inject the liquid chromatograph, record the chromatogram to 2 times of the retention time of the characteristic peak of pazufloxacin mesylate, the HPLC chromatogram is as follo...
Embodiment 2
[0057] Embodiment 2 uses the second mobile phase Pazufloxacin mesylate bulk drug to detect
[0058] The chromatographic conditions are as follows:
[0059] Stationary phase: Octadecylsilane bonded silica gel,
[0060]The second mobile phase: acetonitrile-10% triethylamine methanesulfonate-1M dipotassium hydrogen phosphate-water (45:10:7:138)
[0061] Flow rate: 1.0ml / min,
[0062] Detection wavelength: 254nm,
[0063] Injection volume: 20 μl.
[0064] The detection steps are as follows:
[0065] (1) Prepare the test solution according to the step (1) of Example 1.
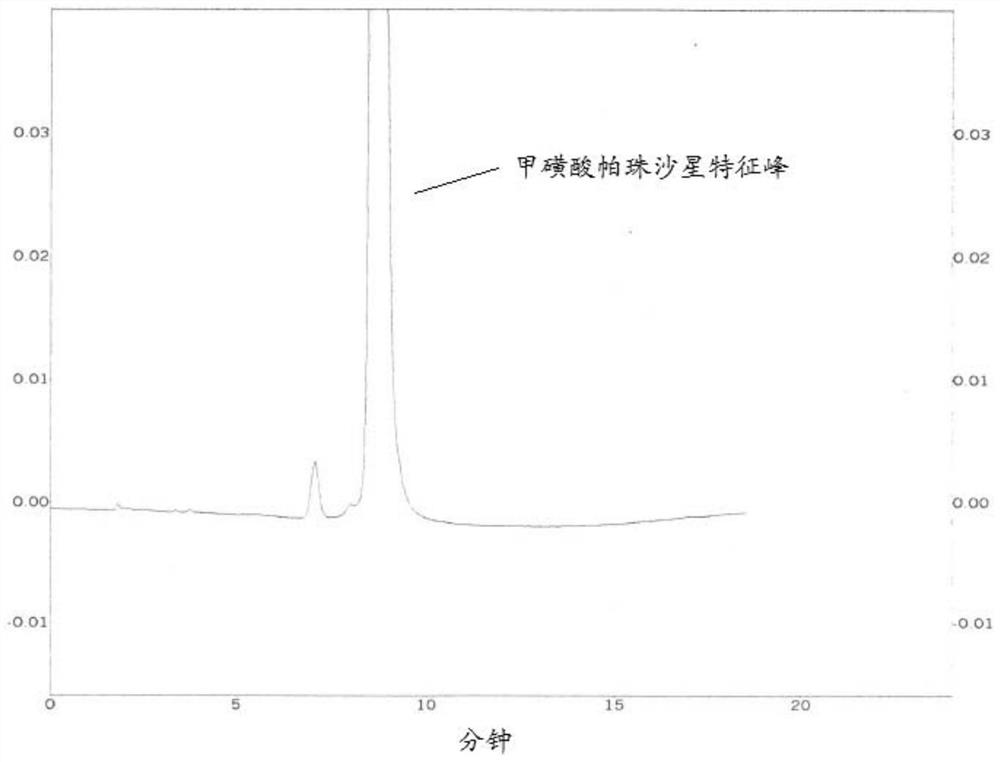
[0066] (2) Under the condition of the second mobile phase, get need testing solution 20 μ l and inject the liquid chromatograph, record the chromatogram to 7 times of the retention time of the characteristic peak of pazufloxacin mesylate, the HPLC chromatogram is as follows Figure 2A shown.
[0067] From Figure 2A It can be seen that, for some related substances with less polarity and peaks after the rete...
Embodiment 3
[0073] Embodiment 3 jointly adopts the first mobile phase and the second mobile phase to detect pazufloxacin mesylate
[0074] The Pazufloxacin Mesylate API (internal batch number 0125) synthesized in-house was tested for related substances.
[0075] The detection steps are as follows:
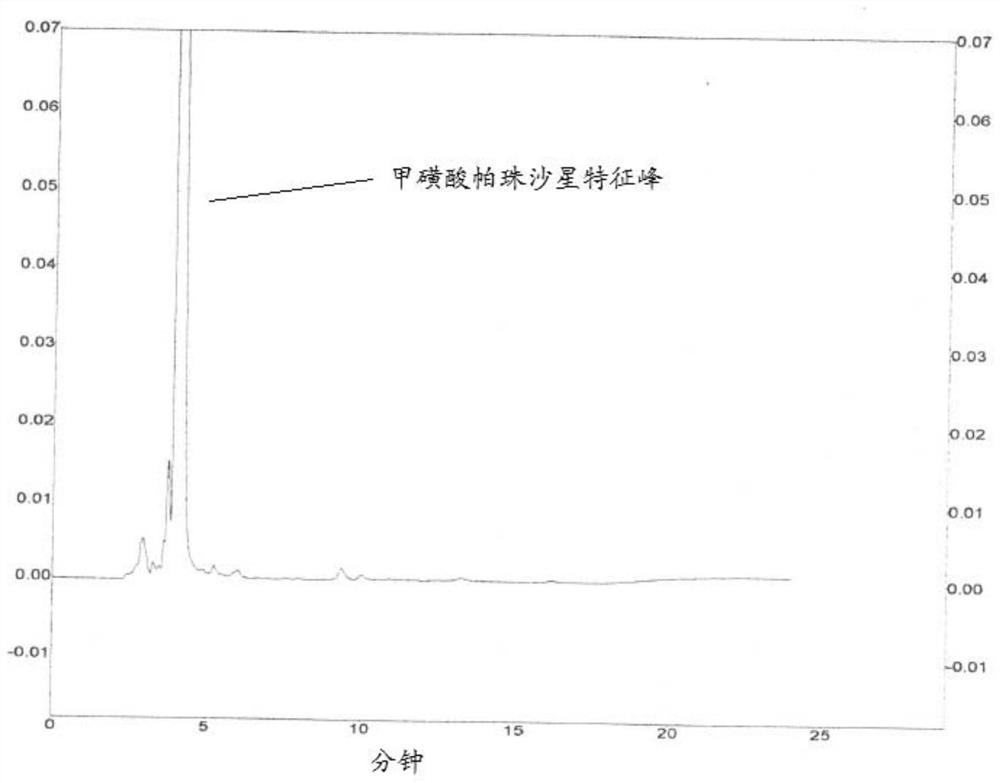
[0076] (1) Get 30 mg of pazufloxacin mesylate crude drug, accurately weighed, put in a 100ml measuring bottle, add the first mobile phase to dissolve and dilute to the scale, shake up, as the test solution; Put 1ml of product solution in a 100ml measuring bottle, dilute to the mark with the first mobile phase, shake well, and use it as a control solution.
[0077] (2) Under the condition of the first mobile phase, get need testing solution and contrast solution and inject high-performance liquid chromatograph respectively, record chromatogram to 2 times of pazufloxacin mesylate characteristic peak retention time, need testing The chromatograms of the solution and the control solution are as ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com