Dry-type immunofluorescence chromatography influenza A/B virus antigen detection kit
An influenza B virus and immunofluorescence technology, which is applied in the field of medical detection and immune analysis, can solve the problems of long detection time, high detection cost, missed detection, etc., shorten the detection window period, reduce the detection limit, and improve stability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
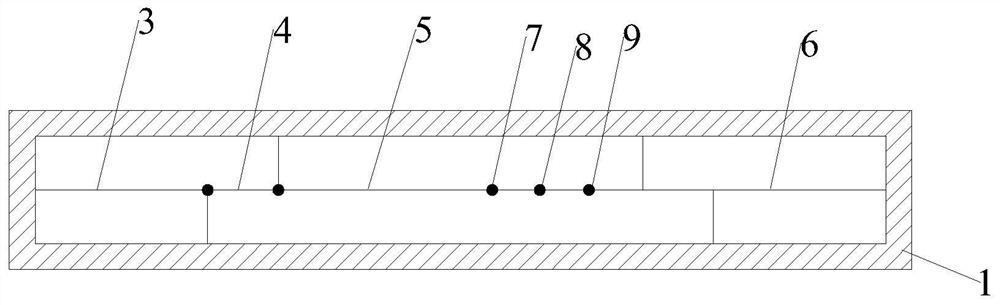
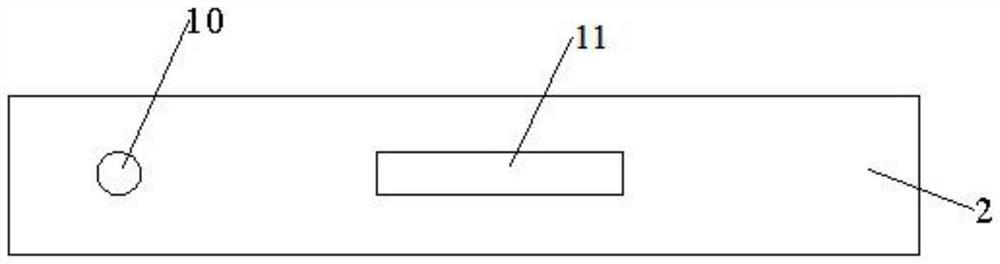
[0038] Such as Figures 1 to 3 As shown, the present invention relates to a dry type immunofluorescence chromatography type A / type B influenza virus antigen detection kit, comprising a detection card, a detection chip and a detection buffer; the detection card comprises a lower cover 1, an upper cover 2 and reagents The lower cover 1 and the upper cover 2 are snap-connected to each other, and a housing cavity is formed between the lower cover 1 and the upper cover 2, the reagent strips are located in the housing cavity, and the upper cover 2 is provided with a sample injection hole 10 and a detection window 11.
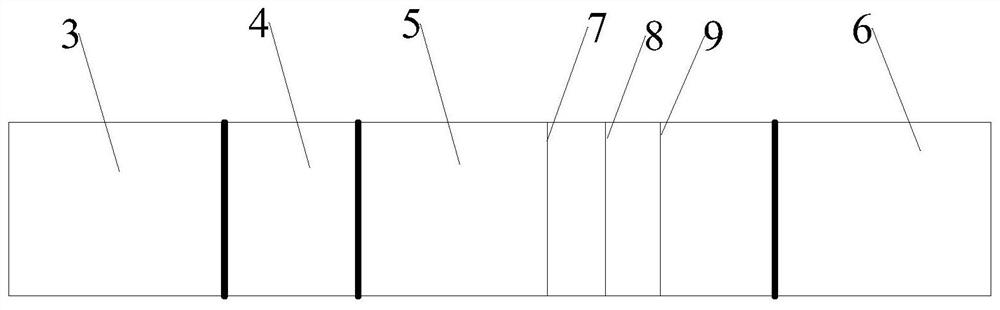
[0039] The reagent strip includes absorbent paper 6, nitrocellulose membrane 5, binding pad 4 and sample pad 3, the absorbent paper 6 is located at one end of the nitrocellulose membrane 5, the sample pad 3 is located at the other end of the nitrocellulose membrane 5, and the binding pad 4 is located at the other end of the nitrocellulose membrane 5. Between the sampl...
Embodiment 2
[0042] A dry immunofluorescence chromatography type A / B influenza virus antigen detection kit, including a detection card, a detection chip and a detection buffer; the detection buffer is 10mM PBS+2wt%BSA+0.02wt%casein+0.3 wt% S9+0.5wt% PC300; the detection card includes reagent strips, and the reagent strips include absorbent paper, nitrocellulose membrane, binding pads and sample pads;
[0043] The nitrocellulose membrane is sequentially coated with quality control line C, detection T1 line and detection T2 line;
[0044] The quality control C line is coated with goat anti-mouse antibody at a concentration of 0.1mg / ml. The goat anti-mouse antibody is diluted with 3wt% sucrose in PBS buffer. After the goat anti-mouse antibody is completely dry, spray 3wt on the quality control C line % sucrose PBS buffer to increase quality control stability; the detection T1 line is coated with a mouse anti-human influenza A virus monoclonal antibody with a concentration of 1.5mg / ml, and the...
Embodiment 3
[0073] A dry immunofluorescence chromatography type A / type B influenza virus antigen detection kit, comprising a detection card, a detection chip and a detection buffer; the detection card comprises a reagent strip, and the reagent strip comprises absorbent paper, nitrocellulose Plain film, binding pad and sample pad; the nitrocellulose membrane is coated with quality control line C, detection T1 line and detection T2 line in sequence; the solid phase of the binding pad is marked with 0.1-0.5 mg / ml fluorescent substance The fluorescent antibody formed by mouse anti-human influenza A / B virus monoclonal antibody; the detection buffer is 10mM PBS+0.5wt%BSA+0.05wt%casein+0.5wt%S9+0.5wt%PC300.
[0074] Further, the fluorescent substance labeling process includes the following steps:
[0075] (1) Place the weighed 1.066g MES in a volumetric flask, add double distilled water to dissolve and adjust the volume to 100mL, prepare 50mM MES, and adjust the pH to 5.8; take the prepared MES ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com