Quality control method and construction method of Tibetan red yeast rice
A technology of quality control method and construction method, which is applied in the field of detection of active ingredients of saffron red yeast rice, to achieve the effects of improving detection efficiency, good repeatability, and good durability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
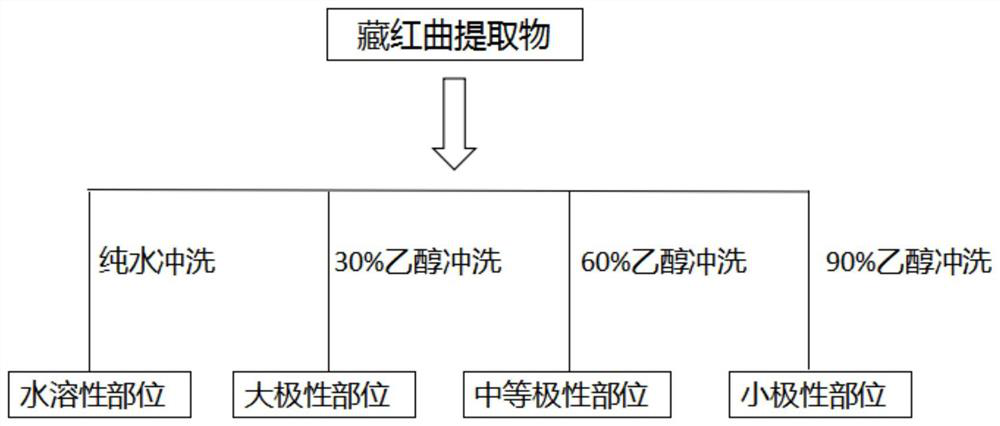
[0089] Embodiment 1 Saffron monascus sample quality control construction method
[0090] Test equipment: KQ-300DE CNC ultrasonic cleaner (power 300W, adjustable frequency, Kunshan Ultrasonic Instrument Co., Ltd.); Milli-Q ultrapure water instrument (Millipore Corporation, USA); electric heating constant temperature blast drying oven (Shanghai New Miao Medical Instrument Manufacturing Co., Ltd.); Alcohol meter; BT25S electronic analytical balance (Beijing Sartorius Instrument System Co., Ltd.); R-210 rotary thin film transevaporator (Swiss BUCHI company); LC-20ATXR Shimadzu liquid chromatography Instrument (Shimadzu Instruments, Japan); ABsicex TOF5600+ mass spectrometer (Aibo Caisi Instrument Company, USA); Disposable sterile syringe (with needle) Shengguang Medical Products Co., Ltd.; Disposable 0.22, 0.45 μm filter membrane Guangzhou City Rui Science Instrument Co., Ltd.; YMC C18 chromatographic column (YMC Technology, Japan);
[0091] Mass spectrometry conditions: electros...
Embodiment 2
[0114] Example 2 Investigation on the Methodology of HPLC Fingerprint of Red Koji Sample
[0115] precision test : according to the quality control construction condition of embodiment 1, accurately draw respectively lovastatin (lactone) reference substance solution (0.1054mg / ml) and lovastatin (acid formula) reference substance solution (0.1243mg / ml) 10 μ L, inject A liquid chromatograph was used for determination, and each reference substance solution was repeatedly injected 6 times, and the peak area was recorded. The precision test results are shown in Table 2.
[0116] Table 2 Precision test results
[0117]
[0118] The results showed that the peak area RSDs of lovastatin (lactone) and lovastatin (acid form) measured above were 0.93% and 0.19%, respectively, both less than 2.0%, indicating that the precision of the system was good.
[0119] repeatability test : get about 0.5g of samples of different batches of red yeast rice, accurately weigh 6 parts, put in a s...
Embodiment 3
[0154] Example 3 HPLC fingerprint analysis of saffron red koji decoction pieces
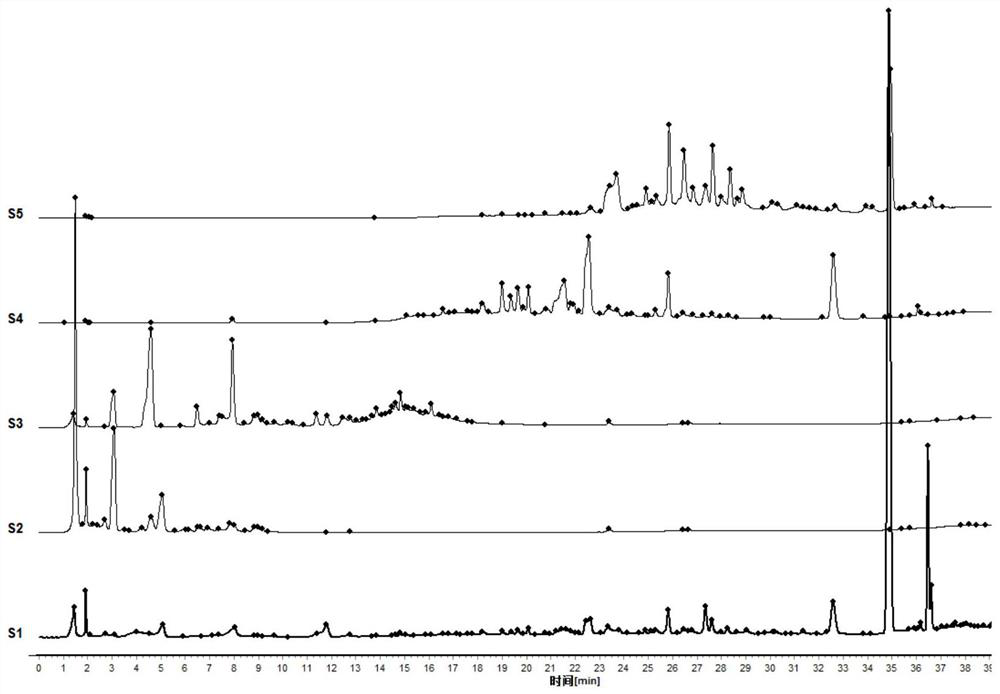
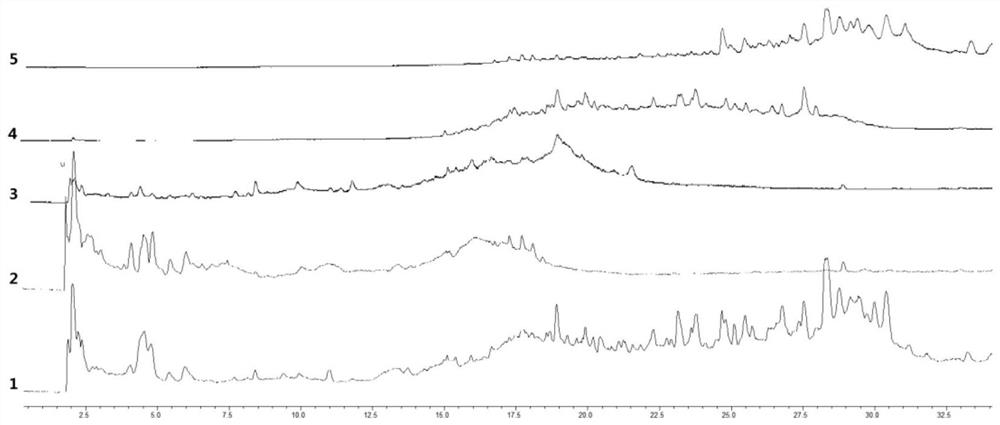
[0155] The detection condition is the same as that in Example 1. Get 14 batches of saffron red koji decoction samples, and get 2 samples for each batch of samples. The chromatographic peaks whose retention time is greater than 1000 in the chromatographic data of 28 samples are automatically integrated in 2 to 38 minutes. The chromatogram of a sample with batch number 20080201 was used as a reference chromatogram, the median generation method was used, and the time width was selected as 0.1min. The chromatographic data of 28 samples were imported into the similarity evaluation system of traditional Chinese medicine chromatographic fingerprints (2012 version), and 6 samples were selected by comparison. Common characteristic component peaks are used as marker peaks for multi-point calibration, and full-spectrum peak matching is performed to obtain peak matching fingerprints ( Figure 5, R: control f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com