Preparation method of HIF-2alpha inhibitor PT2385
A technology of PT2385 and HIF-2, which is applied in the preparation of organic compounds, sulfide preparation, chemical instruments and methods, etc., can solve the problems of unfavorable amplification reactions, low atom economy, lengthy routes, etc., and achieve simple reaction steps and comprehensive synthesis The route is reasonable and the effect of improving the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
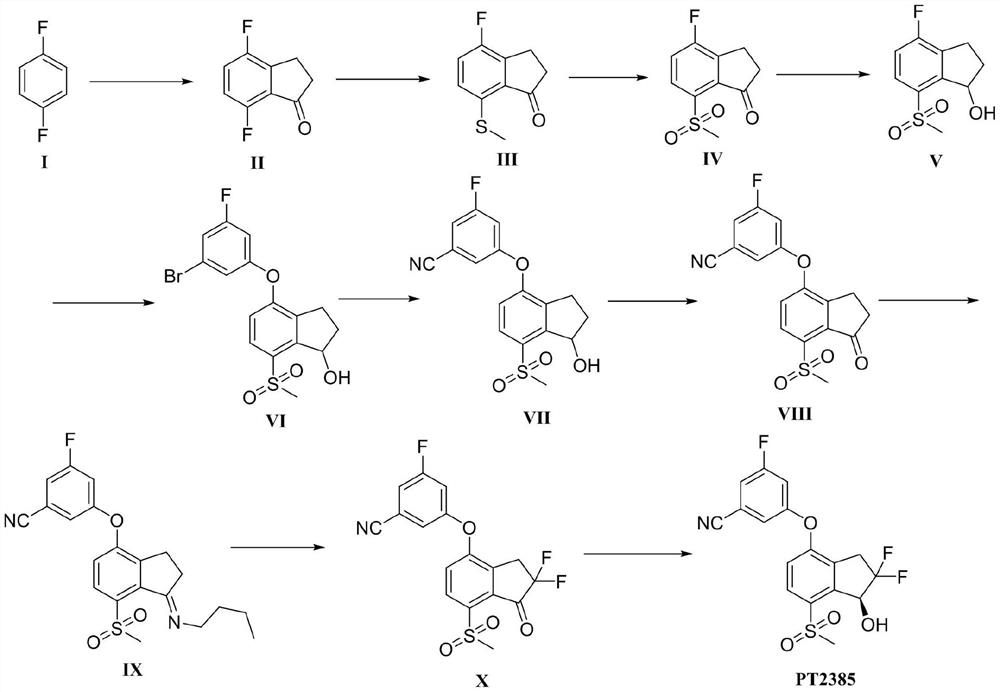
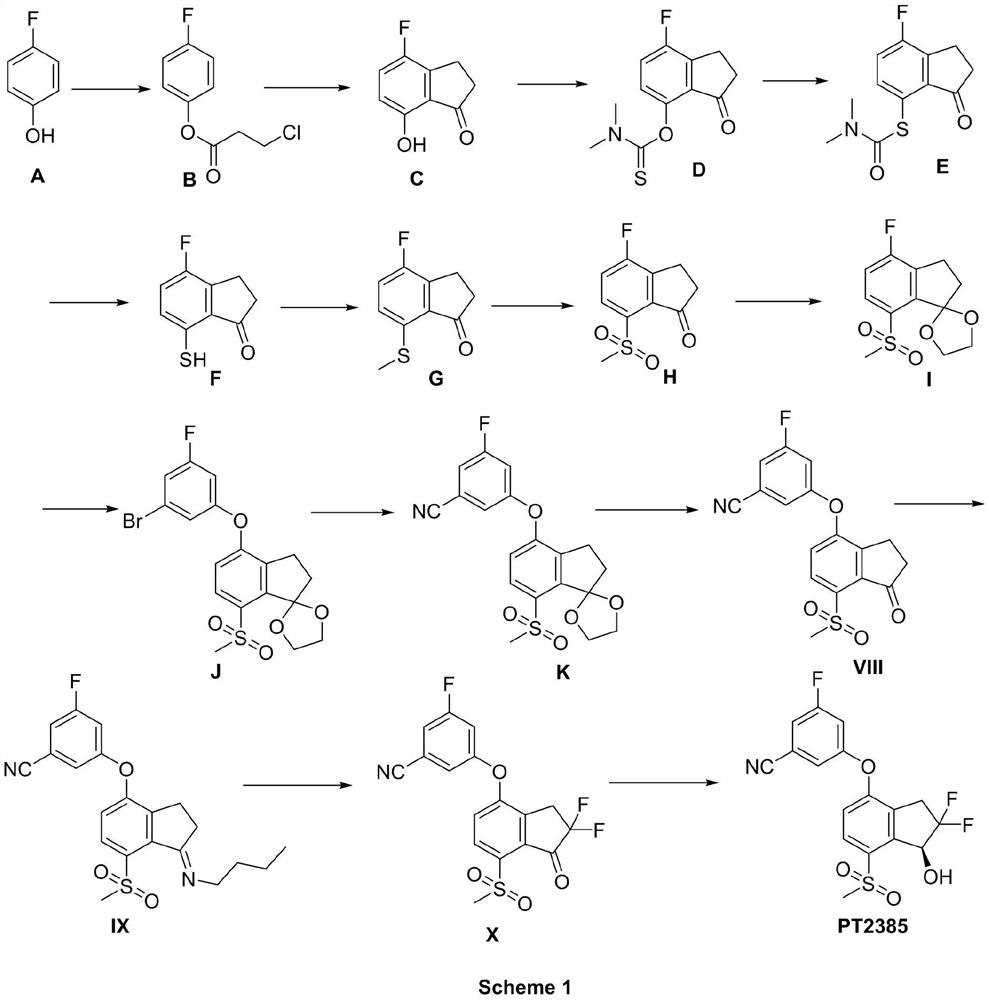
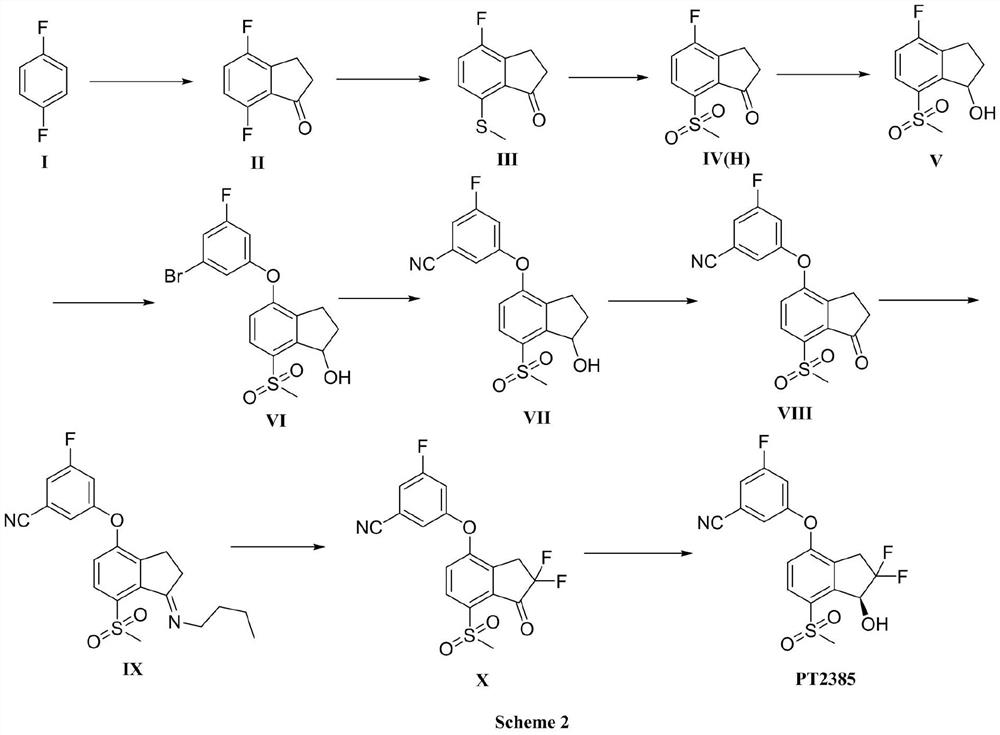
[0048] Embodiment 1 of the present invention provides a kind of preparation method of intermediate II, and its synthetic route is as follows:
[0049]
[0050] Specifically adopt the following method to prepare:
[0051] Compound I (50.0g, 0.44mol), 3-chloropropionyl chloride (55.6g, 0.44mmol) and anhydrous aluminum chloride (116.9g, 0.88mmol) were added into the reaction flask, and then stirred at 120°C for 12h. Return the temperature to room temperature, add water (500mL) and ethyl acetate (500mL) to the reaction flask, separate liquid extraction, dry the organic layer with anhydrous sodium sulfate, filter with suction, and remove the solvent under reduced pressure to obtain 69.8 g of a light yellow solid , yield 94.7%.
[0052] The intermediate II prepared in the present embodiment is identified, and the following results are obtained:
[0053] ESI-MS (m / z): 169.1;
[0054] 1 H NMR (300MHz, CDCl 3 )δ7.32–7.19(m,1H), 7.03–6.96(m,1H), 3.22–3.11(m,2H), 2.83–2.72(m,2H)....
Embodiment 2
[0056] Embodiment 2 of the present invention provides a kind of preparation method of intermediate III, and its synthetic route is as follows:
[0057]
[0058] Specifically adopt the following method to prepare:
[0059] Intermediate II (55.0 g, 0.33 mol) was dissolved in N,N-dimethylformamide (200 mL), and then a 20% aqueous solution of sodium methyl mercaptide (126.1 g, 0.36 mol) was added dropwise at room temperature After reacting for 8h, water (200mL) and ethyl acetate (300mL) were added to the reaction liquid, separated and extracted, the organic layer was dried with anhydrous sodium sulfate, filtered with suction, and the solvent was removed under reduced pressure to obtain 60.3 g of a yellowish-brown solid. The rate is 93.9%.
[0060] The intermediate III prepared in the present embodiment is identified, and the following results are obtained:
[0061] ESI-MS (m / z): 197.2;
[0062] 1 H NMR (300MHz, CDCl 3 )δ7.24(t, J=8.4Hz, 1H), 7.07(dd, J=8.5, 4.0Hz, 1H), 3.1...
Embodiment 3
[0064] Embodiment 3 of the present invention provides a kind of preparation method of intermediate IV, and its synthetic route is as follows:
[0065]
[0066] Intermediate III (60.0 g, 0.31 mol) was dissolved in dichloromethane (300 mL), and m-chloroperoxybenzoic acid with a mass fraction of 85% was added at 0°C, followed by stirring the reaction at 25°C for 18 h, and adding to the reaction solution Add water to the mixture, extract by liquid separation, dry the organic layer with anhydrous sodium sulfate, filter with suction, remove the solvent under reduced pressure, and recrystallize using a mixed solvent of petroleum ether:ethyl acetate (4:1, v:v) to obtain a white solid 61.2 g, yield 87.7%.
[0067] The intermediate IV obtained in the present embodiment is identified, and the following results are obtained:
[0068] ESI-MS (m / z): 229.2;
[0069] 1 H NMR (400MHz, DMSO-d 6 )δ8.10–7.96 (m, 1H), 7.75 (t, J=8.4Hz, 1H), 3.41 (s, 3H), 3.22–3.12 (m, 2H), 2.88–2.76 (m, 2H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com