Rhamnolipid alcohol amide derivative as well as preparation method and application thereof
A technology of rhamnolipid alcohol amide and rhamnolipid is applied in the field of rhamnolipid alcohol amide derivatives and preparation thereof, and can solve the problems of complex rhamnolipid process, complex fermentation liquid composition, and rhamnolipid limitation and other problems, to achieve the effect of improving oil sand cleaning efficiency, excellent oil cleaning effect and surface activity improvement.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
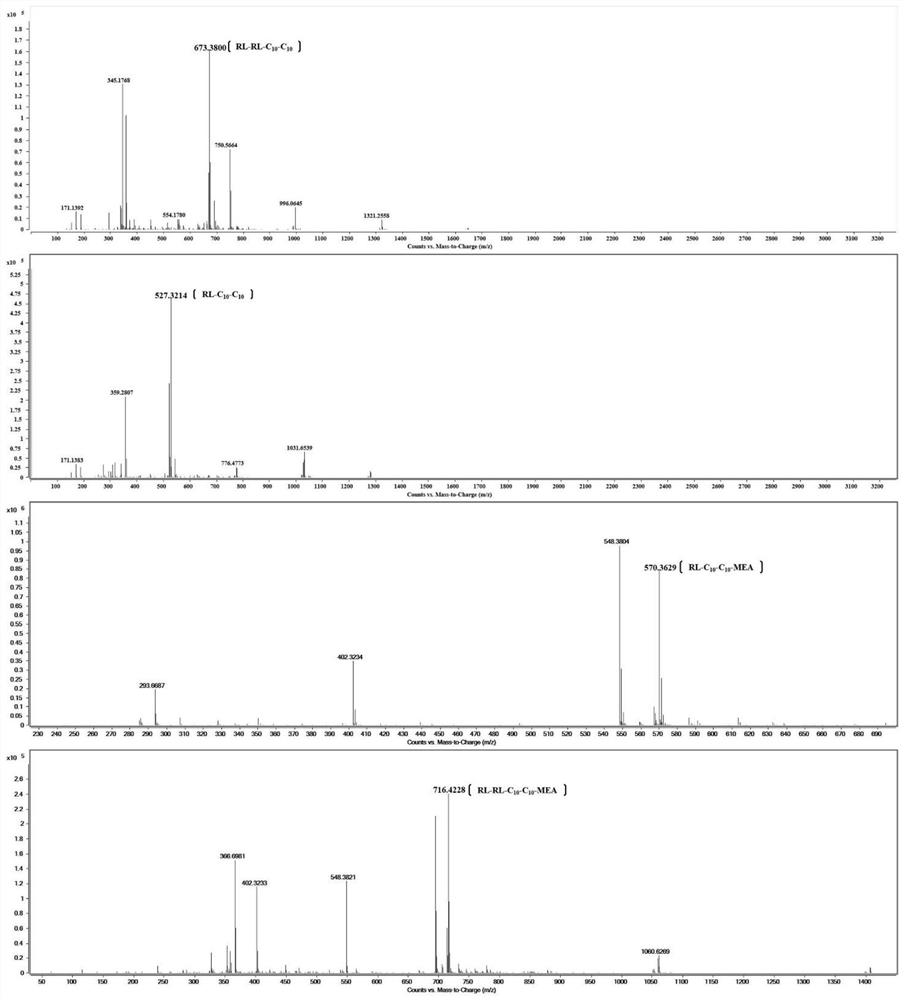
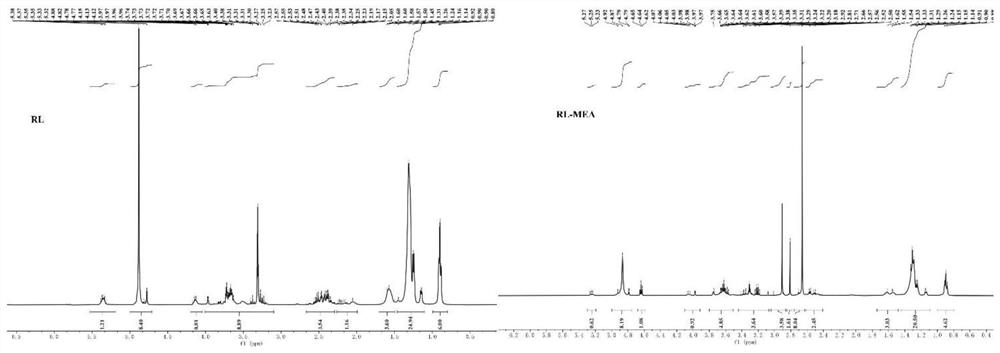
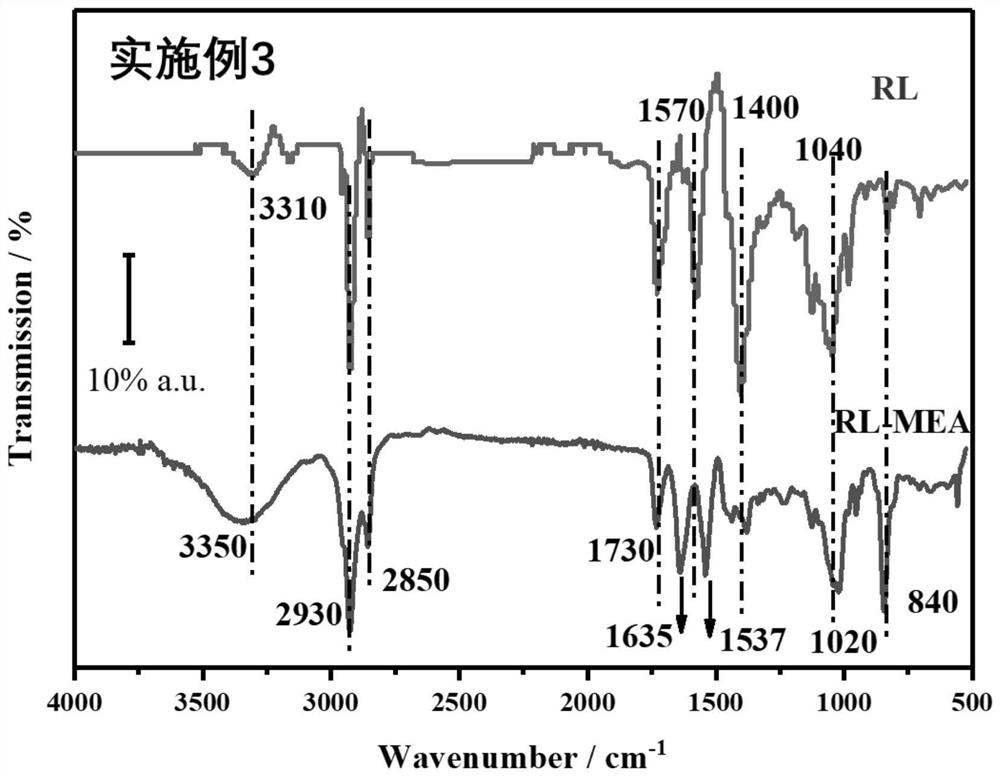
[0039] This example illustrates the synthesis method of the rhamnolipid monoethanolamide of the present invention.
[0040] (1) Dissolve 0.01mol mixed rhamnolipid RL (a mixture of monosaccharide and disaccharide and disaccharide) and 0.03mol triethylamine in 50mL of dichloromethane, and transfer to a three-neck round bottom flask;
[0041] (2) HCTU (0.011mol) pre-dissolved in N,N'-dimethylformamide (DMF) was slowly added dropwise to the three-necked round-bottomed flask described in step (1);
[0042] (3) The round bottom flask in the step (2) is magnetically stirred at 0° C. to fully mix the medicines;
[0043] (4) Add monoethanolamine (0.01mol) dropwise under stirring at 0°C, and then stir the mixture at room temperature for 12h;
[0044] (5) The reaction mixture in step (4) was washed three times with saturated ammonium chloride solution, and extracted with ethyl acetate. The solvents DMF and DCM were removed under reduced vacuum. The residue was poured into 100 mL of ic...
Embodiment 2
[0066] This example illustrates the synthesis method of rhamnolipid diethanolamide of the present invention.
[0067] (1) Dissolve 0.01mol mixed rhamnolipid RL and 0.01mol triethylamine in 50mL methylene chloride, and transfer to a three-necked round bottom flask;
[0068] (2) HCTU (0.012mol) pre-dissolved in N,N'-dimethylformamide (DMF) was slowly added dropwise to the three-necked round-bottomed flask described in step (1);
[0069] (3) The round bottom flask in the step (2) is magnetically stirred at 0° C. to fully mix the medicines;
[0070] (4) Add diethanolamine (0.011mol) dropwise under stirring at 0°C, and then stir the mixture at room temperature for 6h;
[0071] (5) The reaction mixture in step (4) was washed three times with saturated ammonium chloride solution, and extracted with ethyl acetate. The solvents DMF and DCM were removed under reduced vacuum. The residue was poured into 100 mL of ice water and stirred for 10 minutes to obtain a paste. Finally, the pa...
Embodiment 3
[0080] This example illustrates the synthesis method of rhamnolipid monoisopropanolamide of the present invention.
[0081] (1) Dissolve 0.01mol mixed rhamnolipid RL and 0.05mol triethylamine in 50mL methylene chloride, and transfer to a three-necked round bottom flask;
[0082] (2) HCTU (0.01mol) pre-dissolved in N,N'-dimethylformamide (DMF) was slowly added dropwise to the three-necked round-bottomed flask described in step (1);
[0083] (3) The round bottom flask in the step (2) is magnetically stirred at 0° C. to fully mix the medicines;
[0084] (4) While stirring at 0°C, monoisopropanolamine (0.02mol) was added dropwise, and then the mixture was stirred at room temperature for 24h;
[0085] (5) The reaction mixture in step (4) was washed three times with saturated ammonium chloride solution, and extracted with ethyl acetate. The solvents DMF and DCM were removed under reduced vacuum. The residue was poured into 100 mL of ice water and stirred for 10 minutes to obtain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com