Liquid-phase preparation method of sermaglutide side chain
A side chain and liquid phase technology, applied in the field of liquid phase preparation of semaglutide side chains, can solve problems such as difficult product separation, many synthesis steps, and long synthesis cycle, and achieve simplified operation steps, high product yield, The effect of short synthesis cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
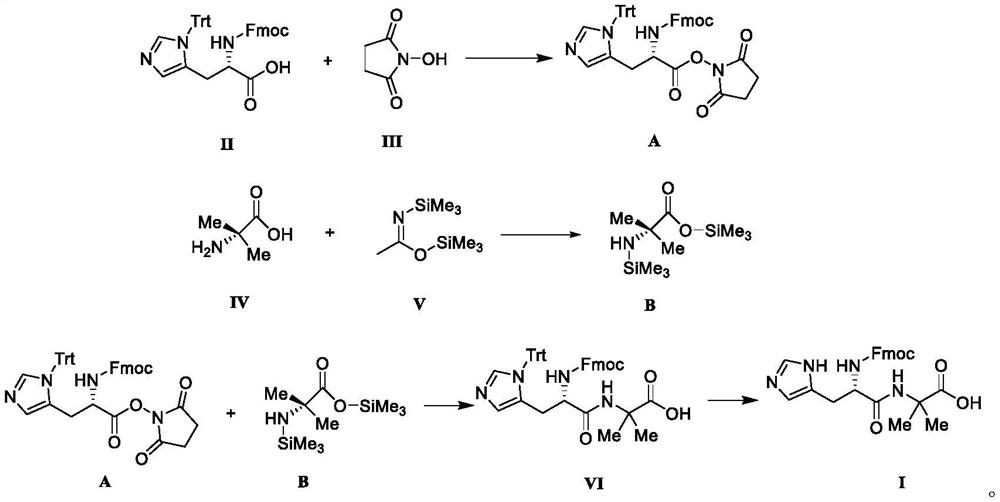
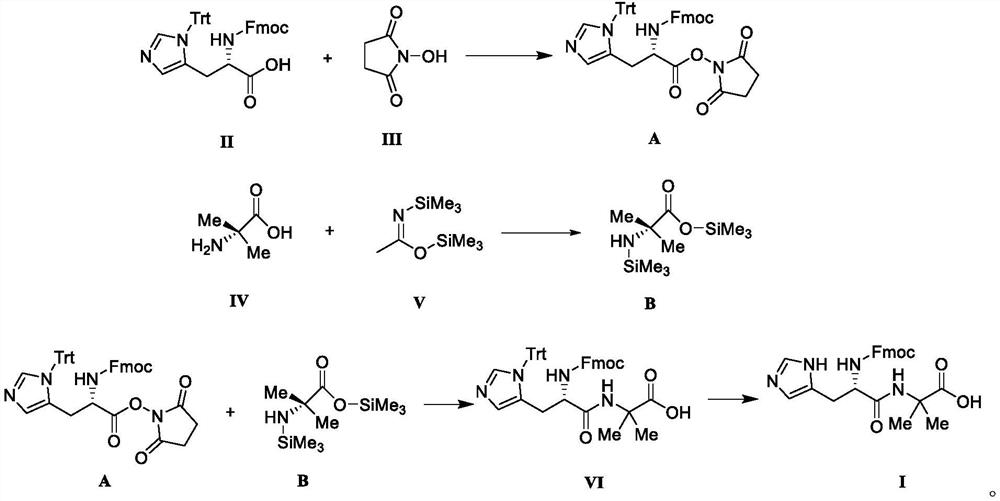
[0049]Put compound II, N-protected-L-histidine (3.10g, 5.0mmol), N-hydroxysuccinimide (1.44g, 12.0mmol) in a reaction flask, add dichloromethane (20mL), stir and dissolve , add dicyclohexylcarbodiimide (2.47g, 12.0mmol) into the reaction solution, stir and react at 35°C for 4h to obtain reaction solution A.
[0050] Put compound IV, 2-aminoisobutyric acid (1.03g, 10.0mmol) in a reaction flask, add dichloromethane (20mL) and compound V, namely N, O-bistrimethylsilylacetamide (4.06g, 20.0 mmol), under the condition of 25°C, stirred and reacted for 14h to obtain reaction solution B.
[0051] Add the reaction solution B to the reaction solution A, stir the reaction at room temperature for 4 h after the addition, and concentrate the reaction solution under reduced pressure until no liquid flows out to obtain a yellow oily liquid. Ethanol (20 mL) was added to the oily liquid, stirred at 5°C, and a white solid was obtained by suction filtration, and vacuum-dried at 40°C to obtain in...
Embodiment 2
[0053] Put compound II, N-protected-L-histidine (3.10g, 5.0mmol), N-hydroxysuccinimide (1.16g, 10.0mmol) in a reaction flask, add tetrahydrofuran (20mL), stir to dissolve, add N,N'-diisopropylcarbodiimide (1.51 g, 12.0 mmol) was added to the reaction liquid, and stirred at 40° C. for 4 h to obtain reaction liquid A.
[0054] Put compound IV, 2-aminoisobutyric acid (1.03g, 10.0mmol) in a reaction flask, add tetrahydrofuran (20mL) and compound V, namely N,O-bistrimethylsilylacetamide (4.06g, 20.0mmol) , Stirring and reacting for 14h at 25°C to obtain reaction solution B.
[0055] Add the reaction solution B to the reaction solution A, stir the reaction at room temperature for 4 h after the addition, and concentrate the reaction solution under reduced pressure until no liquid flows out to obtain a yellow oily liquid. Ethanol (20 mL) was added to the oily liquid, stirred at 5°C, and a white solid was obtained by suction filtration, and vacuum-dried at 40°C to obtain intermediate ...
Embodiment 3
[0057] Put compound II, N-protected-L-histidine (3.10g, 5.0mmol), N-hydroxysuccinimide (1.74g, 15.0mmol) in a reaction flask, add toluene (20mL), stir to dissolve, add Benzotriazol-1-yl-oxytripyrrolidinylphosphonium hexafluorophosphate (6.24 g, 12.0 mmol) was added to the reaction liquid, and stirred at 30° C. for 4 h to obtain reaction liquid A.
[0058] Put compound IV, 2-aminoisobutyric acid (1.03g, 10.0mmol) in a reaction flask, add acetonitrile (20mL) and compound V, namely N,O-bistrimethylsilylacetamide (4.06g, 20.0mmol) , Stirring and reacting for 14h at 25°C to obtain reaction solution B.
[0059] Add the reaction solution B to the reaction solution A, stir the reaction at room temperature for 4 h after the addition, and concentrate the reaction solution under reduced pressure until no liquid flows out to obtain a yellow oily liquid. Ethanol (20 mL) was added to the oily liquid, stirred at 5°C, and a white solid was obtained by suction filtration, and vacuum-dried at ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com