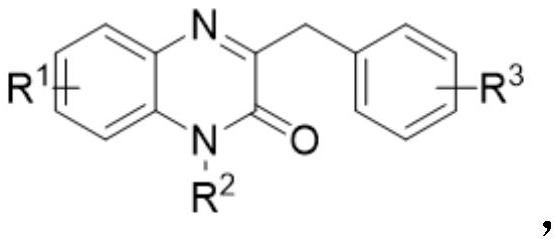
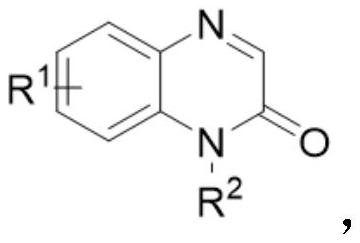
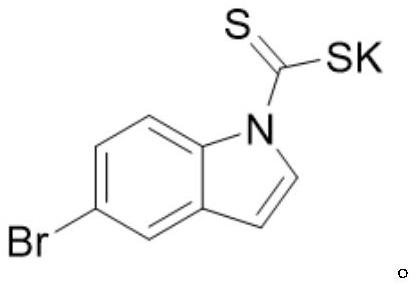
Method for preparing benzylated quinoxalinone compound under visible light catalysis
A technology for benzylated quinoxalinone and quinoxalinone is applied in the field of preparation of benzylated quinoxalinone compounds, can solve the problems of reaction dependence, limited application, few synthesis methods, etc., and achieves mild conditions, Simple operation and high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] In an 8mL reaction flask was added N-methyl-2(1H)quinoxalinone (0.1mmol), benzyl bromide (0.12mmol), sodium acetate (0.12mmol), xanthate (0.01mmol) in 2mL of 1, 2-dichloroethane was irradiated with blue light at 60 degrees Celsius and stirred for 12 hours. After the reaction was completed, the solvent was extracted, dried, and rotary evaporated to remove the solvent. The residue was separated by silica gel column chromatography (petroleum ether:ethyl acetate=10:1) , the yield of the final product was 90%.
[0022] The specific results are as follows:
[0023]
[0024] Yellow solid (22.5 mg, 90%); 1 H NMR (400MHz, Chloroform-d) δ 7.86 (dd, J=7.99, 1.38Hz, 1H), 7.56–7.44 (m, 3H), 7.38–7.24 (m, 4H), 7.26–7.21 (m, 1H) ), 4.27(s, 2H), 3.67(s, 3H); 13 C NMR (101 MHz, Chloroform-d) δ 159.3, 154.7, 137.1, 133.4, 132.8, 130.0, 129.9, 129.5, 128.4, 126.6, 123.6, 113.6, 40.8, 29.1.
Embodiment 2
[0026] In an 8 mL reaction flask was added N-ethyl acetate-2(1H)quinoxalinone (0.2 mmol), benzyl bromide (0.24 mmol), NaOAc (0.24 mmol), xanthate (0.02 mmol) in 4 mL of 1, 2-dichloroethane was irradiated with blue light at 60 degrees Celsius and stirred for 12 hours. After the reaction was completed, the solvent was extracted, dried, and rotary evaporated to remove the solvent. The residue was separated by silica gel column chromatography (petroleum ether:ethyl acetate=10:1) , the yield of the final product was 89%. The specific results are as follows:
[0027]
[0028] Yellow solid (57.2 mg, 89%); 1 H NMR (400MHz, Chloroform-d) δ 7.86 (d, J=7.90Hz, 1H), 7.46 (dd, J=14.08, 7.27Hz, 3H), 7.29 (m, 7.32-7.18Hz, 3H), 7.20 (t, J=7.28Hz, 1H), 7.02(d, J=8.30Hz, 1H), 4.97(s, 2H), 4.27(s, 2H), 4.21(q, J=7.11Hz, 2H), 1.23 (t, J=7.13Hz, 3H); 13 C NMR (101MHz, Chloroform-d) δ 167.1, 159.1, 154.3, 136.8, 132.8, 132.5, 130.3, 130.1, 129.5, 128.4, 126.6, 123.9, 113.0, 62.1, 43.6, 40.6,...
Embodiment 3
[0034] In an 8mL reaction flask was added N-allyl-2(1H)quinoxalinone (0.2mmol), benzyl bromide (0.24mmol), NaOAc (0.24mmol), xanthate (0.02mmol) in 4mL of 1, 2-dichloroethane was irradiated with blue light at 60 degrees Celsius and stirred for 12 hours. After the reaction was completed, the solvent was extracted, dried, and rotary evaporated to remove the solvent. The residue was separated by silica gel column chromatography (petroleum ether:ethyl acetate=10:1) , the yield of the final product was 88%. The specific results are as follows:
[0035]
[0036] Yellow solid (48.5 mg, 88%); 1 H NMR (400MHz, Chloroform-d) δ 7.85 (dd, J=8.00, 1.42 Hz, 1H), 7.46 (m, 3H), 7.29 (m, 3H), 7.25–7.18 (m, 2H), 5.89 ( m, 1H), 5.26–5.21 (m, 1H), 5.16–5.10 (m, 1H), 4.87–4.80 (m, 2H), 4.28 (s, 2H); 13C NMR (101 MHz, Chloroform-d) δ 159.4, 154.3, 137.1, 132.9, 132.6, 130.6, 130.0, 129.8, 129.5, 128.4, 126.6, 123.6, 118.1, 114.1, 44.6, 40.7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com