High-efficiency Golgi apparatus targeting compound and biological application thereof
A technology of aggregation-induced luminescence and Golgi apparatus, which is applied in the field of tumor treatment and biological analysis and detection, and achieves the effect of inhibiting tumor growth, broad application prospects and low cytotoxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: Preparation of compound TPE-PyT-PS (without cyano group in the electron-withdrawing group)
[0041]
[0042] Synthesis of compound 1: in N 2 In the atmosphere, add 1,6-dibromopyrene (5.0g, 13.90mmol), 5-(4,4,5,5-tetramethyl-1,3,2-dioxybenzaldehyde -2-yl)thiophene-2-amino formaldehyde (3.33g, 14.0mmol) and anhydrous potassium carbonate (4.14g), and add 90ml toluene and 15ml water to this system and carry out deoxygenation treatment, add four (three Phenylphosphine) palladium catalyst (0.81 g, 0.70 mmol). The reaction mixture was reacted at 85°C for 18h, and the progress of the reaction was tracked with a TLC plate. After the reaction was completed, the reaction liquid was cooled to room temperature, and the obtained crude product was extracted with water and dichloromethane, and then the solvent was distilled off under reduced pressure. The crude product was subjected to column chromatography using dichloromethane and petroleum ether (DCM:petroleum et...
Embodiment 2
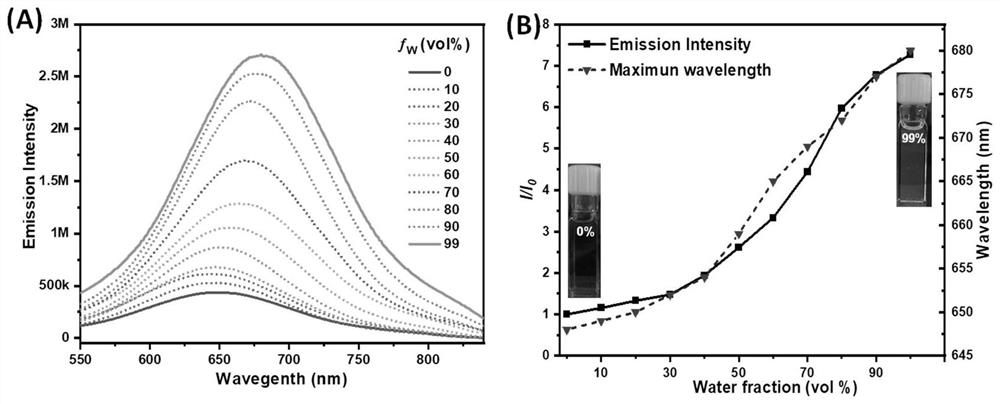
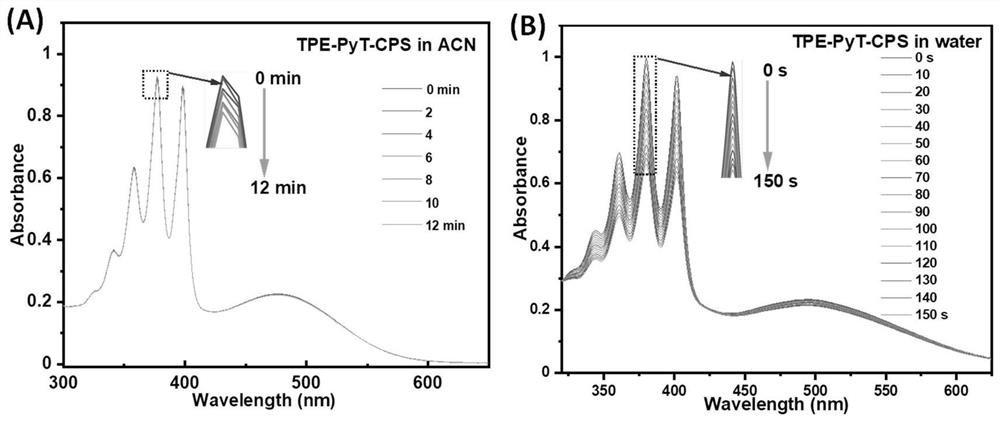
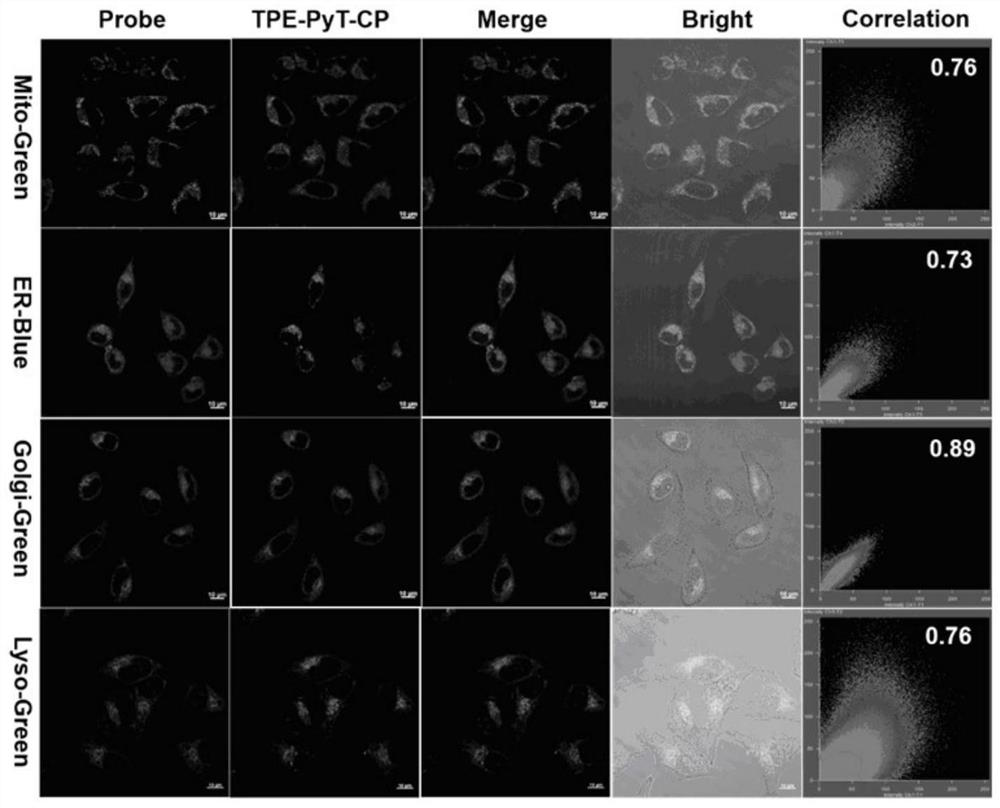
[0045] Embodiment 2: the preparation of compound TPE-PyT-CPS
[0046]
[0047] Synthesis of compound TPE-PyT-CP: under nitrogen protection, compound 2 (147mg, 0.228mmol) and 2-(pyridin-4-yl) acetonitrile (34mg, 0.228mmol) were added in a 50ml two-necked flask, and then added 15ml of ethanol and 4 drops of piperidine. The mixture was heated to 80°C and stirred for 24 hours. After the reaction, it was extracted with dichloromethane and water, and then dried over anhydrous magnesium sulfate. Column separation with DCM (R f =0.4) crude product, 120 mg of red solid was obtained, yield 65.3%. 1 H NMR (400MHz, Chloroform-d) δ8.72(d, J=5.5Hz, 2H), 8.48(d, J=9.2Hz, 1H), 8.27–8.18(m,3H), 8.18–8.12(m, 2H), 8.07–7.99(m, 3H), 7.94(d, J=3.8Hz, 1H), 7.76(d, J=5.0Hz, 2H), 7.55(d, J=3.8Hz, 1H), 7.38( d,J=8.1Hz,2H),7.25–7.13(m,7H),7.07(d,J=8.8Hz,2H),7.01(d,J=8.6Hz,2H),6.75(d,J=8.8 Hz,2H),6.68(d,J=8.7Hz,2H),3.81(s,3H),3.77(s,3H). 13 CNMR(101MHz,Chloroform-d)δ158.27,158.15,148.72,148.71...
Embodiment 3
[0049] Embodiment 3: the preparation of compound HTPA-PyT-CPS
[0050]
[0051] Synthesis of compound 4: in N 2 In the atmosphere, 1-bromooctane (1.93g, 10.0mmol), 4,4'-((4-bromophenyl)azynyl)diphenol (1.89g, 5.07mmol) and Anhydrous potassium carbonate (2.1g, 15mmol), and 90ml DMF was added to the system for deoxygenation treatment, the reaction mixture solution was reacted at 140°C for 18h, and the reaction progress was tracked with a TLC plate. After the reaction was completed, the reaction liquid was cooled to room temperature, and the obtained crude product was extracted with water and dichloromethane, and then the solvent was distilled off under reduced pressure. The crude product was separated by column chromatography using dichloromethane and petroleum ether (DCM:petroleum ether=1:6) to obtain product 4 (2.52 g, 87.1%). 1 H NMR (400MHz, Chloroform-d) δ7.22 (d, J = 8.9Hz, 2H), 7.00 (d, J = 8.6Hz, 4H), 6.90–6.68 (m, 6H), 3.92 (t, J = 6.5Hz, 4H), 1.77(dt, J=14.5, 6.6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com