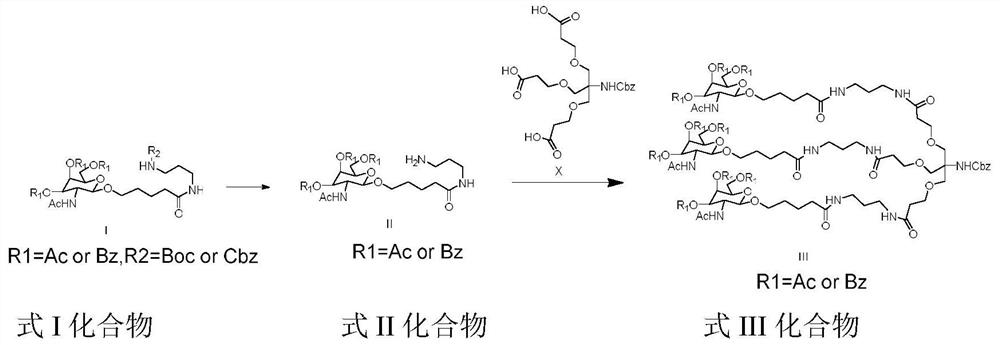
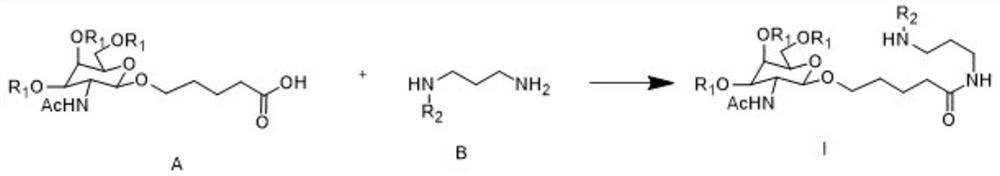
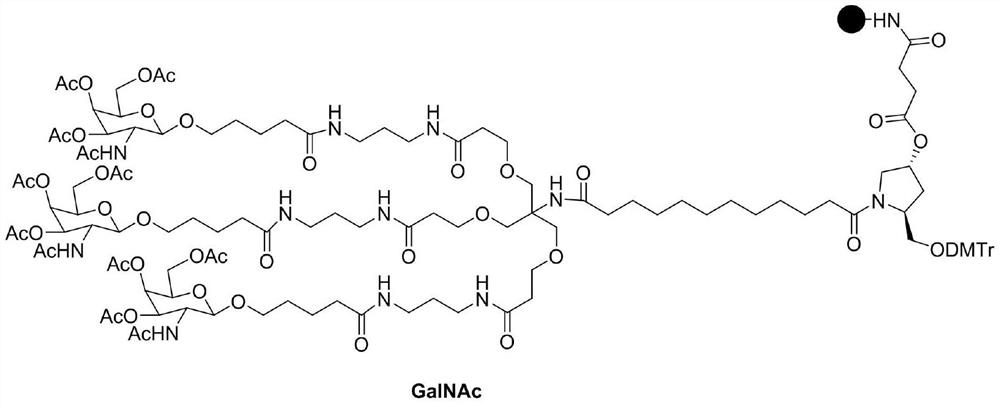
Preparation method of GalNAc intermediate
A technology of intermediates and compounds, which is applied in the field of improving the process and preparing GalNAc intermediates, can solve the problems of long reaction time, low yield, complicated operation, etc., and achieve the effect of convenient purification, high yield, and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Synthesis of I (R1=Ac, R2=Boc)
[0042]
[0043] A (38.0g, 1.0eq) was dissolved in dichloromethane (DCM), CDI (16.3g, 1.1eq) and triethylamine (12.9g, 1.5eq) were added with stirring, and stirred at room temperature for three hours. Then B (16.3g, 1.1eq) was added and reacted overnight at room temperature. After the reaction was completed, the reaction solution was poured into saturated aqueous sodium bicarbonate solution (100 mL), extracted and separated, and the organic phase was collected. Wash the organic phase again with water (100mL), separate the organic phase, add 2N dilute hydrochloric acid to the organic phase, extract and separate layers, wash the organic phase with water (100mL), and finally wash the organic phase with saturated brine (100mL) , collected the organic phase, dried over anhydrous sodium sulfate, filtered, and concentrated to give beige solid I (38.7 g, yield 75.5%).
Embodiment 2
[0044] Embodiment 2: the synthesis of I (R1=Bz, R2=Cbz)
[0045] Dissolve A (63.3g, 0.1mol) in THF1.3L, add 23.0g of EDCI.HCl, 16.2g of HOBt, 50.5g of triethylamine under stirring, stir at room temperature for three hours, then add 2.4g of B6, and heat up to React overnight at 40°C. After the reaction is complete, spin off the solvent, add 500 mL of water and 500 mL of dichloromethane, extract and separate, and collect the organic phase. Wash the organic phase with 500mL of clear water again, separate the organic phase, add 300mL of 2N dilute hydrochloric acid to the organic phase, extract and separate layers, wash the organic phase with 500mL of clear water, and finally wash with 500mL of saturated saline, and dry over anhydrous sodium sulfate. Filtration and concentration gave light yellow solid I (61.4 g, yield: 74.6%).
Embodiment 3
[0046] Embodiment 3: the synthesis of I (R1=Bz, R2=Boc)
[0047]Dissolve A (6.33g, 0.01mol) in 80mL of 2-methyltetrahydrofuran, add 5.69g of HBTU and 1.93g of DIPEA under stirring, and stir at room temperature for three hours. Subsequently, 2.09 g of B was added, and the temperature was raised to 40 to react overnight. After the reaction is complete, add 50 mL of water, extract and separate the liquid, add 30 mL of 2N dilute hydrochloric acid to the organic phase, extract and separate layers, wash the organic phase with 50 mL of water, and finally wash with 50 mL of saturated saline, dry over anhydrous sodium sulfate, filter, and concentrate , to obtain light yellow solid I (5.63g, yield: 71.3%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com