Indirect ELISA method of clostridium perfringens beta1 toxin antibody
A technology of Clostridium perfringens and toxin, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problem of lack of detection products of toxin, and achieve the effect of strong specificity, good stability and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1: Expression of soluble recombinant Clostridium perfringens β1 toxin protein:
[0053] According to the sequence of Clostridium perfringens β1 toxin gene reported in the literature, design primers
[0054] Forward(5`-3`)CGGAATTCCATATGGATATAGGCAAAACTACTACTAT
[0055] Reverse(5`-3`)GAATGCGGCCGCAATAGCTGTTACTTTATG
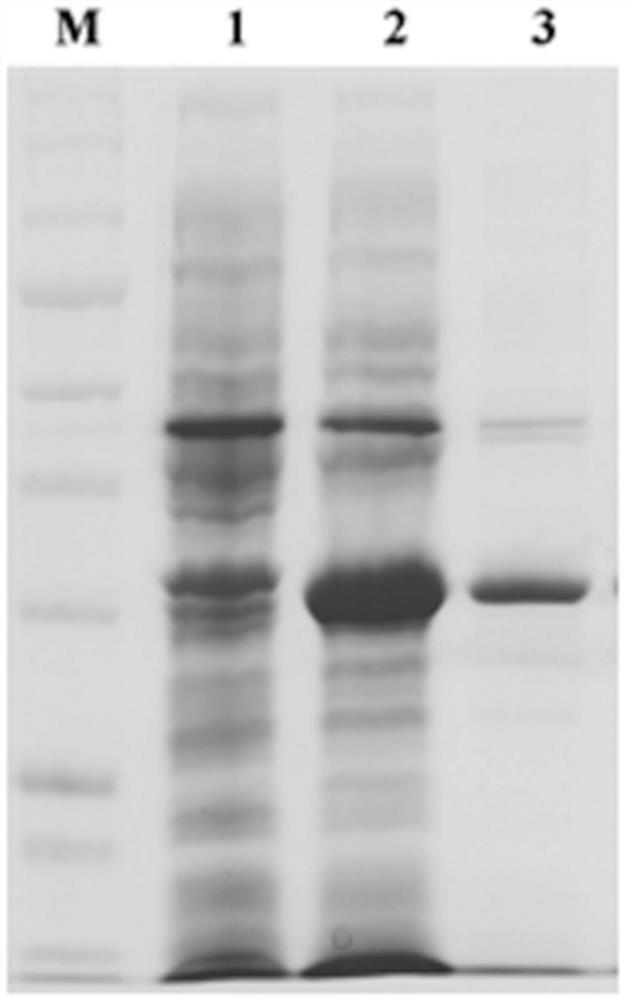
[0056] The cpb1 gene was amplified from the C-type standard strain of Clostridium perfringens, constructed into pTIG-Trx vector, transformed into BL21(DE3) strain, and labeled as BL21(DE3)pTIG-cpb1.
[0057] Take the BL21(DE3)pTIG-cpb1 glycerol frozen strain, streak it on the LB solid plate containing 100 μg / mL ampicillin, pick a single colony the next day and insert it into 10 mL LB liquid medium containing 100 μg / mL ampicillin for culture When the OD600 of the bacterial solution reaches above 1.0, inoculate 1% inoculum in 200 mL LB liquid medium containing 100 μg / mL ampicillin and culture for 3 hours, then induce expression at 20 °C and 0.5 mM IPTG ...
Embodiment 2
[0058] Example 2: Purification of soluble recombinant Clostridium perfringens β1 toxin protein:
[0059] Centrifuge to collect 800mL of recombinant expression cells after induction, wash once with PBS, add 20mM Tris-HCL (pH 8.0) buffer to resuspend 10mL of cell pellet, add 100μg / mL lysozyme, 1‰ TritonX-100, 0.01mM PMSF, Sonicate for 10min; centrifuge at 10,000rmp for 10min, take the precipitate and wash it once with 10mL of 20mM Tris-HCL (pH 8.0) buffer containing 0.2M urea; pH 8.0) denaturing buffer, incubate at 37°C for 2 hours to completely dissolve and denature the precipitate; transfer the dissolved and denatured protein solution to the dialysis bag, and add 150 mL of urea to the liquid outside the dialysis bag in sequence with a concentration gradient of 1.5M and 1M respectively , 0.5M, 0M 20mM Tris-HCL (pH 8.0) refolding buffer, each concentration gradient treatment for 6h.
Embodiment 3
[0060] Example 3: Determination of Coating Conditions for Soluble Clostridium perfringens β1 Toxin Protein Antigen:
[0061] Using the matrix titration method, the β1 toxin recombinant protein was used as the coating antigen, and the β1 toxin recombinant protein was diluted with the coating buffer, and the concentrations in each well were 2 μg / mL, 5 μg / mL, 8 μg / mL, and 10 μg / mL. Dilute in mL, add 100 μL to each well of the microtiter plate, and coat overnight at 4°C; use 5% skimmed milk powder, use purified McAb1K19 as the primary antibody, HRP-goat anti-mouse IgG as the secondary antibody, OPD as the chromogenic solution, 2M H 2 SO 4 The solution is the stop solution, and the absorbance value is detected by measuring the OD450nm value.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com