Injectable naproxen preparation and application thereof
A naproxen and injection technology, applied in the field of injectable naproxen preparations, can solve problems such as turbid insoluble matter, reduce the rapid bioavailability of active ingredients, avoid secondary pollution, facilitate large-scale production, The effect of using less material
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The preparation method of described injectable naproxen preparation, comprises the steps:
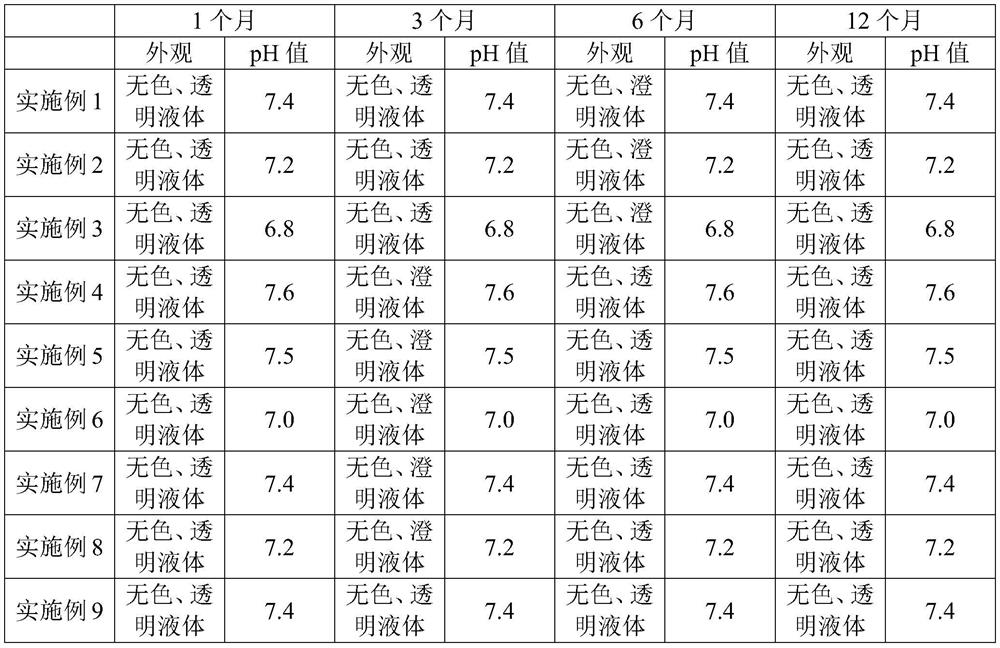
[0039] S1. Accurately weigh 2.48g sodium phosphate dodecahydrate and add it to 800mL water for injection to dissolve, then add 5.00g naproxen to continue dissolving, then add 7.5g sodium chloride to dissolve to obtain a mixed solution;
[0040] S2, using 1.0mol / L sodium hydroxide, 1.0mol / L potassium hydroxide or 1.0mol / L hydrochloric acid to adjust the pH value of the mixed solution obtained in step S1 to 7.4, and then adding water for injection to dilute to 1000g to obtain a diluted solution;
[0041] S3. After filtering the diluted solution obtained in step S2, sub-package, and obtain a colorless and clear injectable naproxen preparation with a naproxen concentration of 250 mg / 50 ml after sterilization.
Embodiment 2
[0043] The preparation method of described injectable naproxen preparation, comprises the steps:
[0044] S1. Accurately weigh 1.88g sodium phosphate dodecahydrate and add it to 800mL water for injection to dissolve, then add 2.5g naproxen to continue dissolving, then add 8.0g sodium chloride to dissolve to obtain a mixed solution;
[0045] S2, using 1.0mol / L sodium hydroxide, 1.0mol / L potassium hydroxide or 1.0mol / L hydrochloric acid to adjust the pH value of the mixed solution obtained in step S1 to 7.2, and then adding water for injection to dilute to 1000g to obtain a diluted solution;
[0046] S3. After filtering the diluted solution obtained in step S2, sub-package, and obtain a colorless and clear injectable naproxen preparation with a naproxen concentration of 250 mg / 100 ml after sterilization.
Embodiment 3
[0048] The preparation method of described injectable naproxen preparation, comprises the steps:
[0049] S1. Accurately weigh 4.13g sodium phosphate dodecahydrate and add it to 800mL water for injection to dissolve, then add 2.50g naproxen to continue dissolving, then add 7.0g sodium chloride to dissolve to obtain a mixed solution;
[0050] S2, using 1.0mol / L sodium hydroxide, 1.0mol / L potassium hydroxide or 1.0mol / L hydrochloric acid to adjust the pH value of the mixed solution obtained in step S1 to 6.8, and then adding water for injection to dilute to 1000g to obtain a diluted solution;
[0051] S3. After filtering the diluted solution obtained in step S2, sub-package, and obtain a colorless and clear injectable naproxen preparation with a naproxen concentration of 250 mg / 100 ml after sterilization.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com