Method for synthesizing thioether from thiophenol and aryl halide
A halogenated aromatic hydrocarbon and thiophenol technology, applied in the fields of organic chemistry and photocatalysis, can solve the problems of high synthesis cost, restrict large-scale production, etc., and achieve the effects of low production cost, improved conversion rate and no metal residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
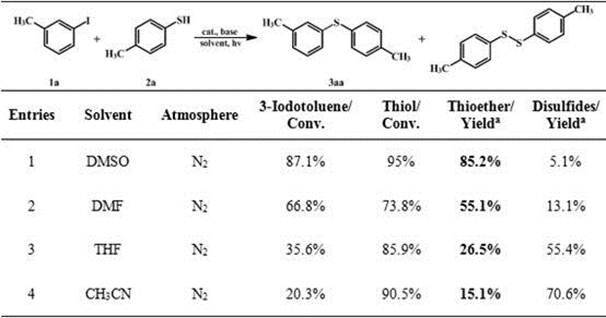
[0025] Using p-toluenethiophenol and 3-methyliodobenzene as substrates, Pd / ZnIn 2 S 4 is a photocatalyst, in N2 Under the atmosphere, dimethyl sulfoxide (DMSO), dimethylformamide (DMF), tetrahydrofuran (THF) and acetonitrile (CH 3 CN) as a solvent, KOH was added, and the reaction was carried out at room temperature and under visible light, and the specific reaction conditions were as described above. The reaction results are shown in the following table, wherein when DMSO is used as the solvent, the conversion rate and thioether yield are significantly higher than other solvents.
[0026] Table 1 Solvent to Pd / ZnIn 2 S 4 Influence of Photocatalytic Coupling Reaction of Thiophenols with Halogenated Iodobenzenes
[0027]
Embodiment 2
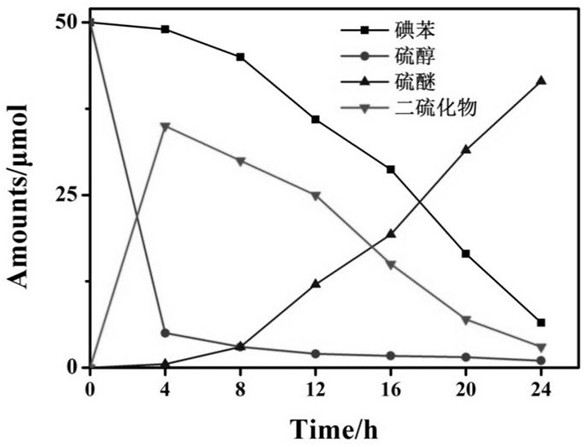
[0029] Using p-toluenethiophenol and 3-methyliodobenzene as substrates, Pd / ZnIn 2 S 4 is a photocatalyst, in N 2 Under the atmosphere, dimethyl sulfoxide was used as solvent, KOH was added, and the specific reaction conditions were as described above, and the time distribution diagram of the substrate and different products was made, such as figure 1 shown. The conversion rate of iodobenzene and the yield of target product aryl sulfide increased with time, and the thiol was almost completely converted into disulfide or thiophenol anion at the beginning of the reaction. The yield of disulfides decreased with time, indicating that disulfides would further participate in the reaction, resulting in the formation of sulfide products.
Embodiment 3
[0031] in N 2 Under the atmosphere, using dimethyl sulfoxide as a solvent, under the conditions of room temperature, light and alkali, the halogenated aromatic hydrocarbon is 3-iodotoluene, the reaction substrate thiophenol is explored, and the specific reaction conditions are as described above. The experimental results show that thiophenols with electron-withdrawing group EWG and electron-donating group EDG attached to the benzene ring can react with 3-iodotoluene, but the electronic effect and steric effect have certain influence on the yield of the target product.
[0032] Table 2 Substrate expansion reactions of thiophenols with different substituents
[0033]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com