Preparation method of 2, 4-diamino-6-chloropyrimidine
A chloropyrimidine and diamine-based technology, applied in the field of pharmaceutical raw material preparation, can solve the problems of high cost, low equipment utilization rate, complicated procedures and the like, and achieve the effects of high temperature stability, safe and gentle quenching, and obvious cost advantage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Weigh 2,4-diamino-6-hydroxypyrimidine (12.6g) into the three-necked flask, add POCl 3 (53.5g) was added into the reaction flask, the temperature was raised to 105°C, and stirred for 6h. After the reaction, the excess POCl was evaporated 3 , lowered the temperature to 30-40°C, slowly added ethanol (37ml) dropwise, raised the temperature to reflux reaction for 2h after dropping, lowered the temperature, and added ethyl acetate (96ml). Cool and stir for 2h, filter. 17.8 g of DACP hydrochloride was obtained.
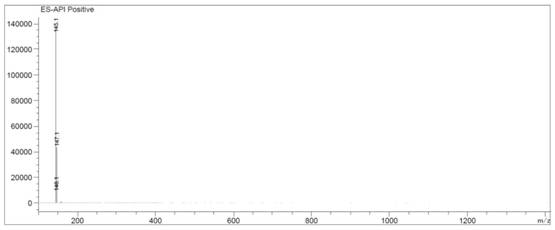
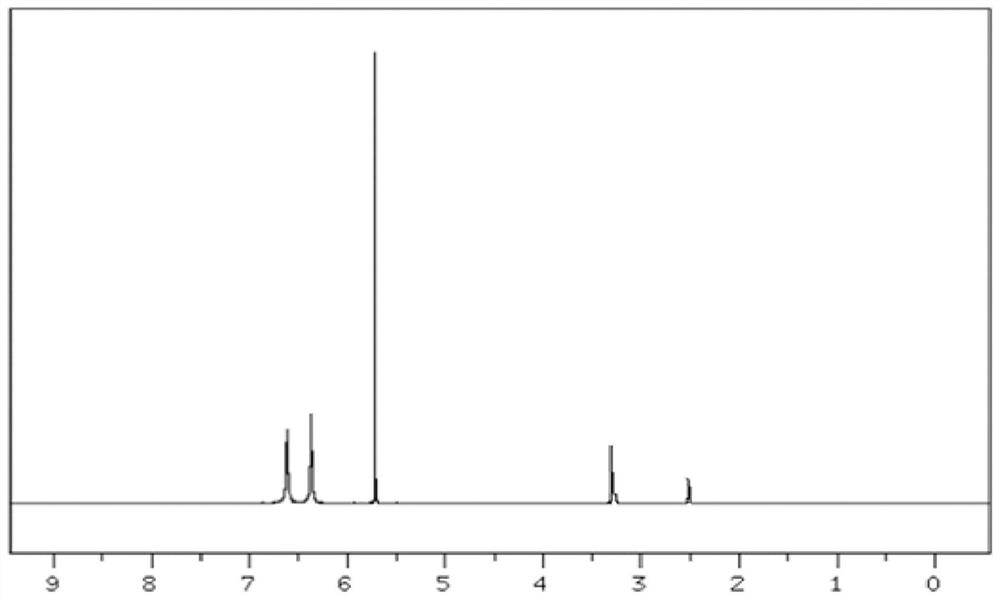
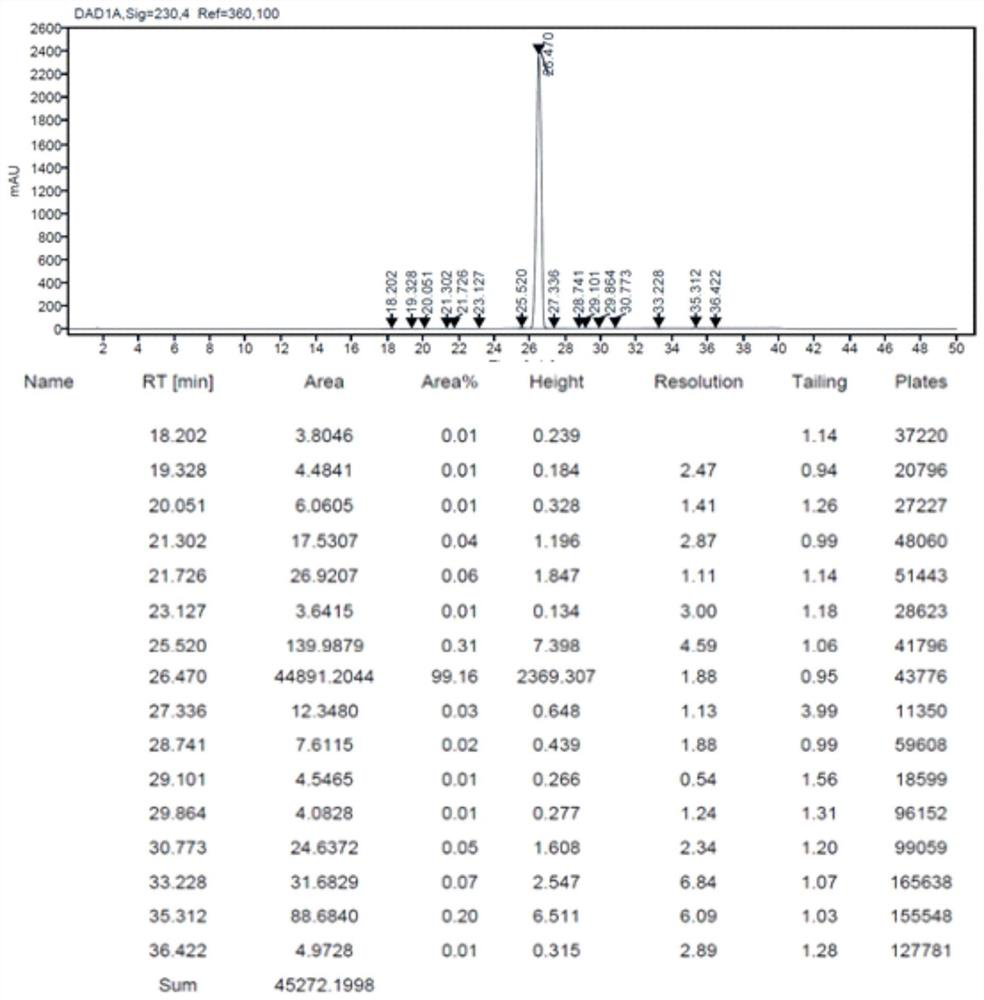
[0028] Add DACP hydrochloride into water (40ml), heat up to 70°C to dissolve, neutralize with ammonia water to pH 6-7, extract with ethyl acetate, dry and concentrate to obtain DACP pure product 11.8g, yield 82.0%, HPLC: 99.2% . [M+H+]=145.1, H-NMR (DMSO-d6): δ6.64 (S, NH2), δ6.42 (S, NH2), δ5.73 (S, Ar-H).
Embodiment 2
[0030] Weigh 2,4-diamino-6-hydroxypyrimidine (12.6g) into the three-necked flask, add POCl 3 (76.5g) was added into the reaction flask, the temperature was raised to 105°C, and stirred for 6h. After the reaction, the excess POCl was evaporated 3 , lower the temperature to 30-40°C, slowly add ethanol (60ml) dropwise, after the dropping, raise the temperature to reflux for 2h, cool down, and add acetone (60ml). Cool and stir for 2h, filter. 15.4 g of DACP hydrochloride was obtained.
[0031] DACP hydrochloride was added to water (40ml), heated to 70°C to dissolve, neutralized with ammonia water to pH 6-7, extracted with ethyl acetate, dried and concentrated to obtain 10.5g of pure DACP with a yield of 73.0%.
Embodiment 3
[0033] Weigh 2,4-diamino-6-hydroxypyrimidine (12.6g) into the three-necked flask, add POCl 3 (76.5g) was added into the reaction flask, the temperature was raised to 105°C, and stirred for 6h. After the reaction, the excess POCl was evaporated 3 , the temperature was lowered to 30-40°C, methanol (40ml) was slowly added dropwise, after the dropwise temperature was raised to reflux for 2h, the temperature was lowered, and acetonitrile (60ml) was added. Cool and stir for 2h, filter. 16.2 g of DACP hydrochloride was obtained.
[0034] DACP hydrochloride was added to water (40ml), dissolved at 70°C, neutralized with ammonia water to pH 6-7, extracted with ethyl acetate, dried and concentrated to obtain 11.1g of DACP with a yield of 77.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com