Beta-lactam compound and application and preparation method thereof
A compound and technology of general formula, applied in the field of beta-lactam compounds and their preparation, to achieve the effects of low drug resistance and excellent antibacterial activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
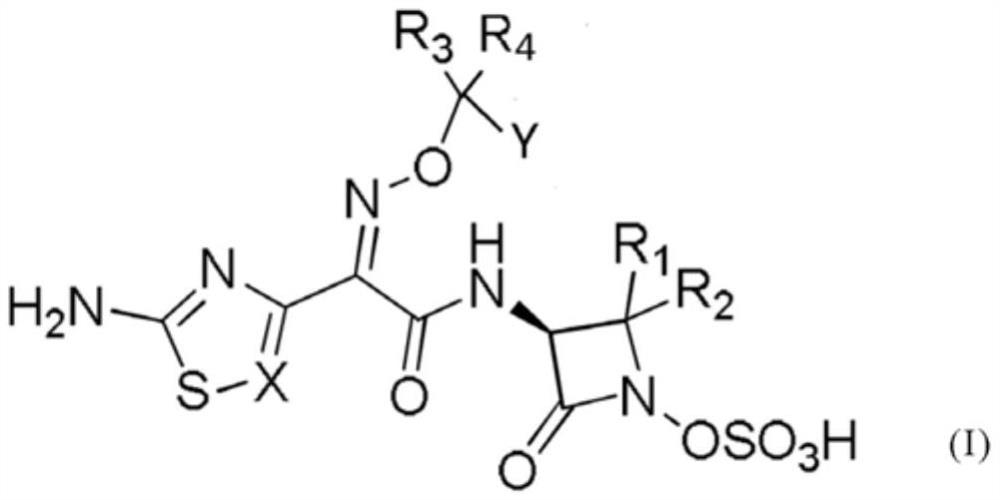
[0093] In another aspect of the present invention, the preparation method of the compound represented by the following general formula (I) is provided, which comprises:
[0094] Step a:
[0095] By reacting the compound shown in the following formula (1) and the compound shown in the formula (2), the compound shown in the formula (3) is generated;
[0096]
[0097] In the above formulas, PG represents a protecting group;
[0098] R 1 , R 2 Each independently represents a hydrogen atom, a C1-10 straight-chain or branched alkyl group optionally with substituents, or both form a cycloalkyl group with a ring carbon number of 3 to 8; R 3 , R 4 Each independently represents a hydrogen atom, a C1-10 linear or branched alkyl group optionally having a substituent, a C6-12 aryl group optionally having a substituent, or both together form a ring with 3 to 3 carbon atoms. 8 cycloalkyl group; X represents C or N; Y represents a straight-chain or branched alkenyl or alkynyl group or...
Embodiment
[0192] Hereinafter, the present invention will be described in more detail using production examples, test examples, and the like, but the present invention is not limited to these examples.
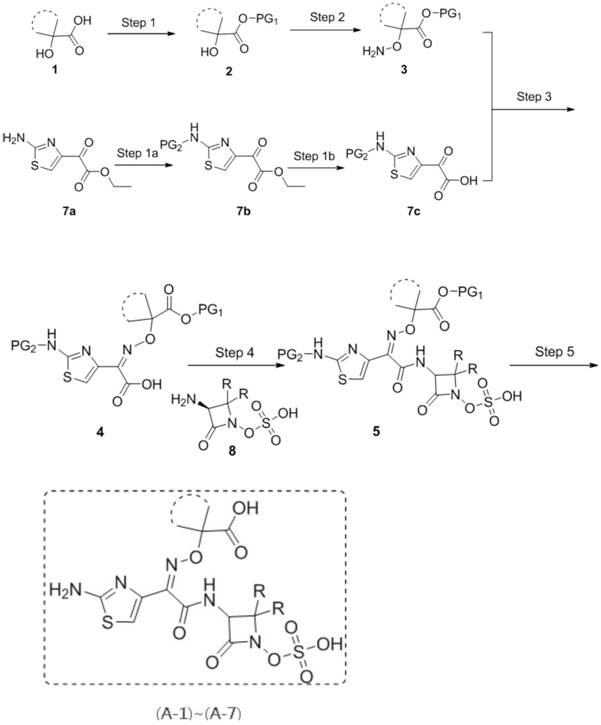
[0193] The specific synthetic route of compound (A-1)~(A-7) is as follows:
[0194]
manufacture example 1
[0196] Production Example 1: Synthesis of Compound (A-1)
[0197]
[0198] Step 1a (Step 1a): Compound 7a (1.0mmol) was dissolved in DMF (10.0v / g), triethylamine (2.0mmol) was added, triphenylchloromethane (1.2mmol) was added in batches, and the reaction was stirred at room temperature After 6 hours, TLC monitoring, after the reaction is complete, add water / ethyl acetate for extraction, combine the organic phases, dry and concentrate, and separate by column chromatography to obtain the target compound 7b.
[0199] Step 1b (Step 1b): Dissolve compound 7b (1.0mmol) in 1,4-dioxane (5v / g) / water (5v / g), stir at room temperature, add sodium hydroxide (5.0mmol), Continue to stir the reaction, TLC monitors, the raw material disappears, distill out 1,4-dioxane under reduced pressure, adjust the pH to 2-3, stir for 10 minutes, filter, wash the filter cake with water until the filtrate is neutral, collect and dry the filter cake to obtain compound 7c.
[0200] Step 1 (Step 1): compo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com