Synthesis method of 2, 4, 6-tribromophenol
A synthesis method, the technology of tribromophenol, applied in the field of compound synthesis, can solve problems such as difficult transportation and storage, potential safety hazards in production, and easy corrosion of metals, and achieve the effects of low production cost, high safety, and low pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
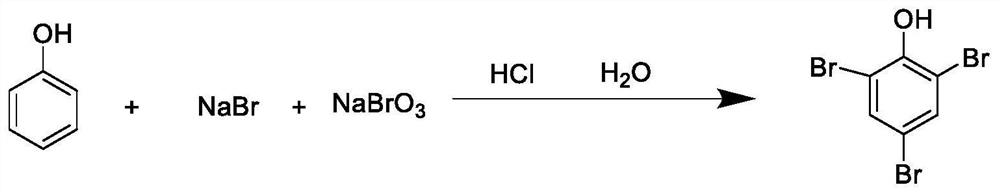
[0043] This embodiment is a kind of synthetic method of 2,4,6-tribromophenol, such as figure 1 Shown, this synthetic method comprises the steps:
[0044] (a) phenol and metal bromide are dissolved in water to obtain a mixed solution of phenol and metal bromide, which is denoted as the first mixed solution, wherein the concentration of phenol in the mixed solution of phenol and metal bromide is 1mmol / ml, and the metal bromide The concentration of compound is 2mmol / ml; Metal bromide is sodium bromide;
[0045] (b) Under stirring conditions, add bromate solution and hydrochloric acid dropwise into the first mixed solution and react at 20-45°C for 3h, wherein the volume ratio of bromate solution, hydrochloric acid and the first mixed solution is 5:3:10; bromate solution concentration is 2mmol / ml; hydrochloric acid concentration is 36%; bromate is sodium bromate;
[0046] (c) After the reaction is completed, add sodium sulfite to the reaction solution until the light brown color ...
Embodiment 2
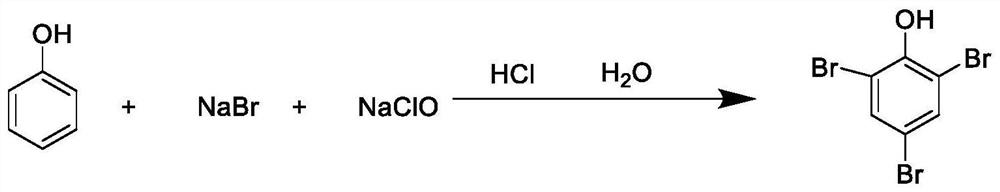
[0057] The present embodiment is a synthetic method of 2,4,6-tribromophenol, and the synthetic method comprises the following steps:
[0058] (a) dissolve phenol and metal bromide in water to obtain a mixed solution of phenol and metal bromide, denoted as the first mixed solution, wherein the concentration of phenol in the mixed solution of phenol and metal bromide is 1.2 mmol / ml, and the metal The concentration of bromide is 2.4mmol / ml; the metal bromide is potassium bromide;
[0059] (b) under stirring conditions, add bromate solution and hydrochloric acid dropwise to the first mixed solution and react at 20 to 45 ° C for 2 h, wherein the volume ratio of bromate solution, hydrochloric acid and first mixed solution is 5:2:12; bromate solution concentration is 2.2mmol / ml; hydrochloric acid concentration is 30%; bromate is potassium bromate;
[0060] (c) treat that reaction finishes, in reaction solution, add sodium sulfite to light brown color disappears completely in reactio...
Embodiment 3
[0065] The present embodiment is a synthetic method of 2,4,6-tribromophenol, and the synthetic method comprises the following steps:
[0066] (a) dissolving phenol and metal bromide in water to obtain a mixed solution of phenol and metal bromide, denoted as the first mixed solution, wherein the concentration of phenol in the mixed solution of phenol and metal bromide is 0.8 mmol / ml, and the metal The concentration of bromide is 1.6mmol / ml; the metal bromide is sodium bromide;
[0067] (b) under stirring conditions, add bromate solution and hydrochloric acid dropwise to the first mixed solution and react at 20~45°C for 4h, wherein the volume ratio of bromate solution, hydrochloric acid and first mixed solution is 5:4:8; bromate solution concentration is 1.8mmol / ml; hydrochloric acid concentration is 40%; bromate is sodium bromate;
[0068] (c) treat that reaction finishes, in reaction solution, add sodium sulfite to light brown color disappears completely in reaction solution,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com