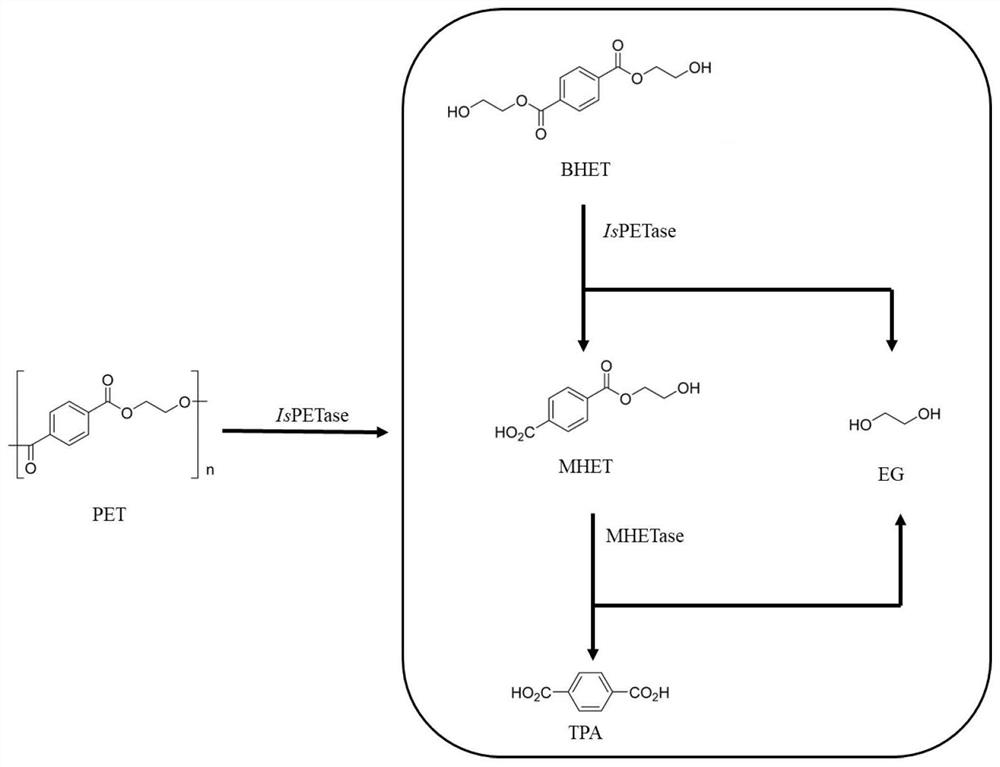
PET hydrolase IsPETase mutant enzyme, coding gene and engineering bacterium
A technology of mutant enzymes and hydrolytic enzymes, which is applied in the fields of enzyme engineering and bioengineering, can solve the problems of low stability, enzyme activity, and limited applications, and achieve the effect of improving hydrolysis efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Rational Design of Mutation Sites of PET Hydrolase IsPETase
[0035] (1) Signal peptide prediction of PET hydrolase IsPETase:
[0036] Use the SignalP-5.0Server online server to analyze whether there is a signal peptide and a suitable cleavage site in the original sequence (SEQ ID NO.3) of the PET hydrolase IsPETase.
[0037] (2) Simulation of the spatial structure of PET hydrolase IsPETase
[0038] Based on the IsPETase enzyme (PDB: 5XG0) analyzed by Xu Han et al. (PDB: 5XG0) as a model (SEQ ID NO.3), its structure was found to have a set of typical features:
[0039] ①IsPETase enzyme consists of three polypeptide chains, which are represented as A, B and C chains;
[0040] ②Belongs to the α / β hydrolase superfamily and presents a typical α / β hydrolase folding structure. The central β-sheet structure is formed by 9 mixed β strands (β1-β9), surrounded by 7 α-helices (α1-β9). α7) surround;
[0041] ③The catalytic triad (S131-H208-D177) is strictly conserved and locate...
Embodiment 2
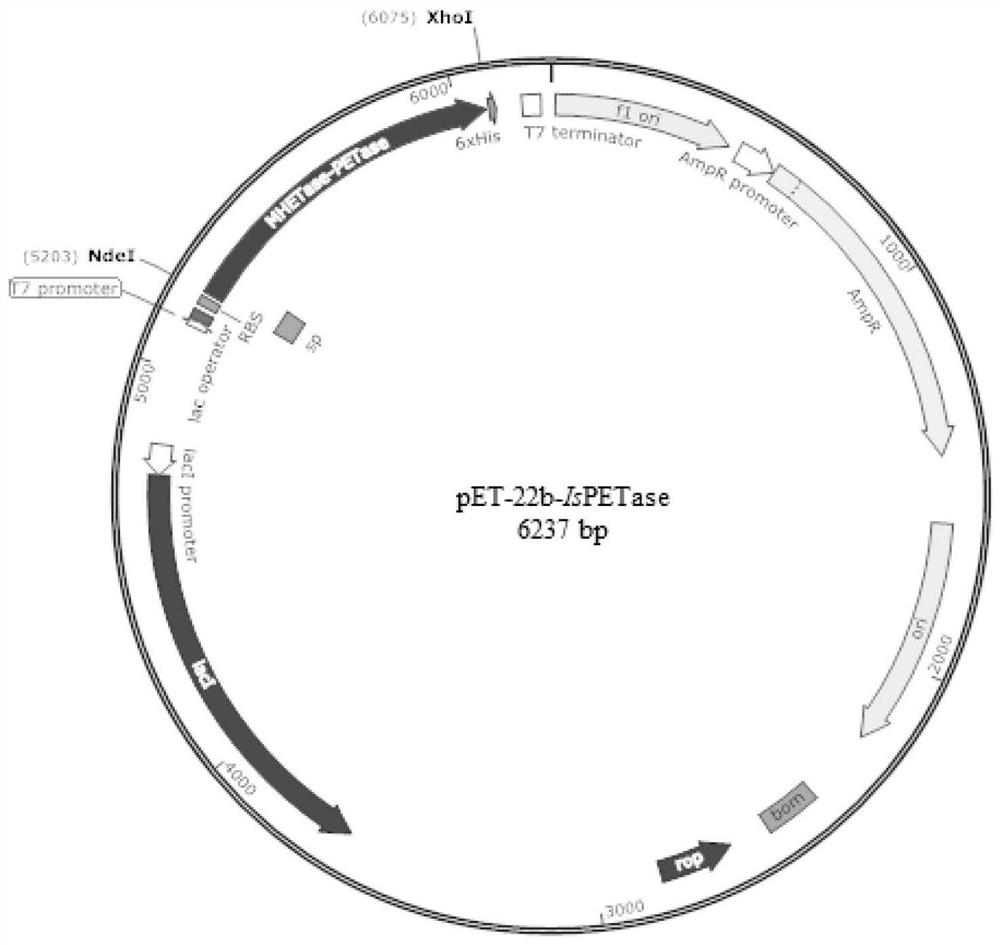
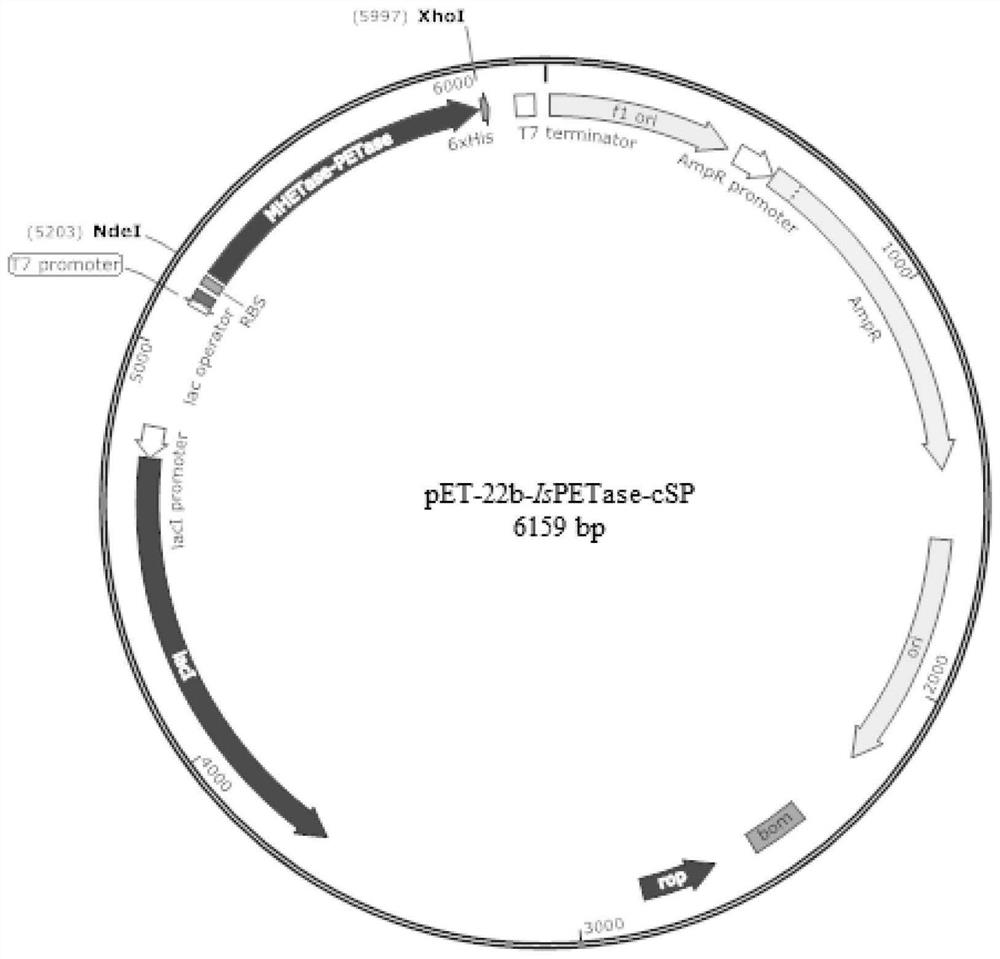
[0051] Preparation of recombinant plasmids pET-22b-IsPETase-cSP and pET-22b-MHETase-cSP
[0052] The construction of the recombinant plasmid pET-22b-IsPETase was obtained by linking the wild-type PET hydrolase IsPETase gene (SEQ ID NO.4) to the pET-22b plasmid.
[0053] The construction of the recombinant plasmid pET-22b-MHETase was obtained by linking the wild-type MHETase gene (SEQ ID NO.8) to the pET-22b plasmid. The amino acid sequence of the wild-type MHETase enzyme is described in SEQ ID NO.7.
[0054] Wherein, the source of the wild-type PET hydrolase IsPETase gene: Ideonella sakaiensis (GI: 1028065175).
[0055] Among them, the source of the wild-type MHETase gene: Ideonella sakaiensis (GI: 1027923624).
[0056] The pET-22b plasmid is commercially available.
[0057] The preparation method of recombinant plasmid (recombinant plasmid pET-22b-IsPETase-cSP and pET-22b-MHETase-cSP), concrete steps are as follows:
[0058] According to the requirements of the seamless re...
Embodiment 3
[0068] Contains a mutant enzyme encoding PET hydrolase IsPETase (IsPETase R280A / S121K -cSP) gene recombinant plasmid (abbreviation: recombinant plasmid containing mutant gene) preparation
[0069] Based on the mutation site rationally designed in Example 1, the primers for site-directed mutagenesis were designed and synthesized as follows:
[0070] R280A-F: 5'-GAACAGCACCGCGGTGTCGGACTTCCGCACCGC (mutation introduced at position 280) (SEQID NO.13)
[0071] R280A-R: 5'-GTCCGACACCGCGGTGCTGTTCGGGTTCTCGC (mutation introduced at position 280) (SEQ ID NO.14)
[0072] S121K-F: 5'-GACCAGCCGAAAAGCCGCTCGTCGCAGCAG (mutation introduced at position 121) (SEQ ID NO.15)
[0073] S121K-R: 5'-GAGCGGCTTTTCGGCTGGTCGAGCGTGGAG (mutation introduced at position 121) (SEQ ID NO.16)
[0074] According to the requirements of the seamless recombination kit, the recombinant plasmid containing the mutant gene was constructed by seamless connection and reverse PCR technology, which is mainly divided into t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com