D-psicose-3-epimerase active aggregate as well as preparation method and application thereof
A technology of epimerase and allulose, which is applied in the field of enzyme engineering, can solve the problems of low recycling rate, poor thermal stability, unfavorable cost control of D-psicose, etc., to reduce production costs and improve Yield and yield, effects of loss avoidance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
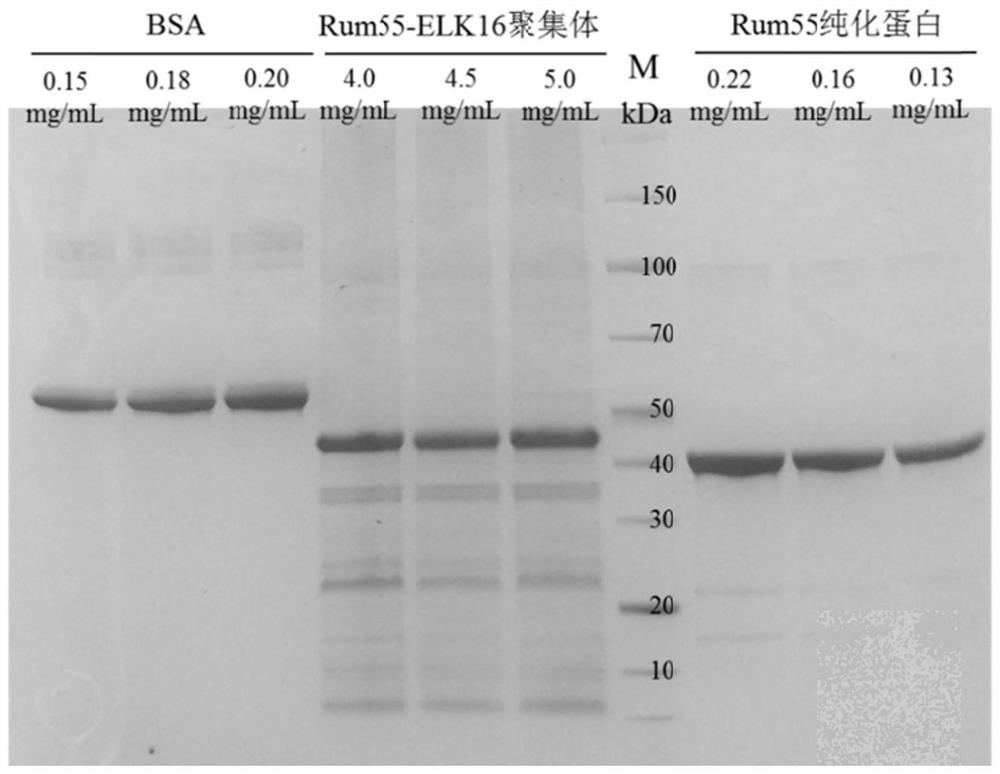
[0073] Example 1 Obtaining of D-psicose-3-epimerase active aggregates (obtaining a fragment containing D-psicose-3-epimerase-connecting peptide-self-aggregating short peptide )
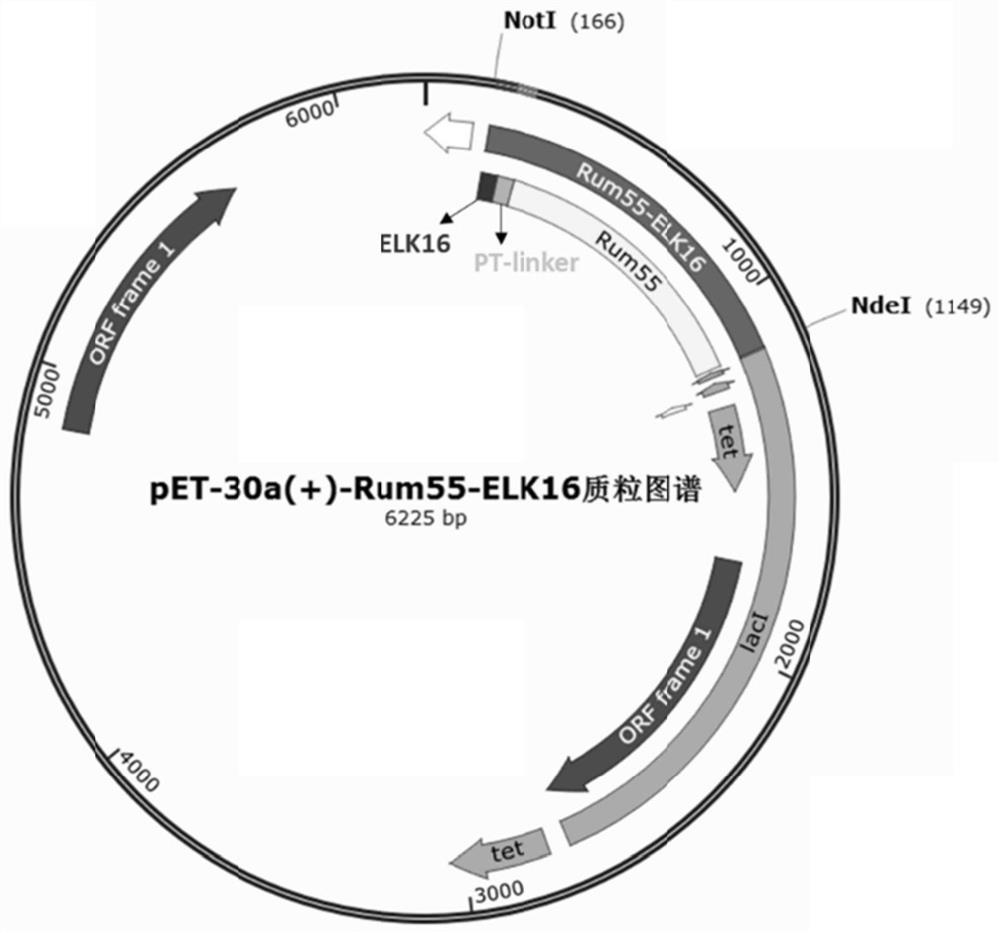
[0074] 1. Obtain the Rum55-PT-ELK16 target fragment containing pET30a vector and restriction site sequence
[0075] According to the connecting peptide PT-linker (SEQ ID NO.18), self-aggregating short peptide ELK16 (SEQ ID NO.19), D-psicose-3-epimerase Rum55 (SEQ ID NO.17), and the gene sequence of the pET30a vector (purchased from Solarbio, Cat. No. P3120), respectively designed primers to amplify the PT-ELK16 fragment and the Rum55-PT fragment, and used the above two amplified fragments as templates to design The primers of the dot sequence were used for overlap PCR amplification, and finally the target fragment of Rum55-PT-ELK16 containing the pET30a vector and restriction site sequence was obtained. The details are as follows:
[0076] (1) PT-ELK16 fragment PCR amplification
[0077] Amplified...
Embodiment 2
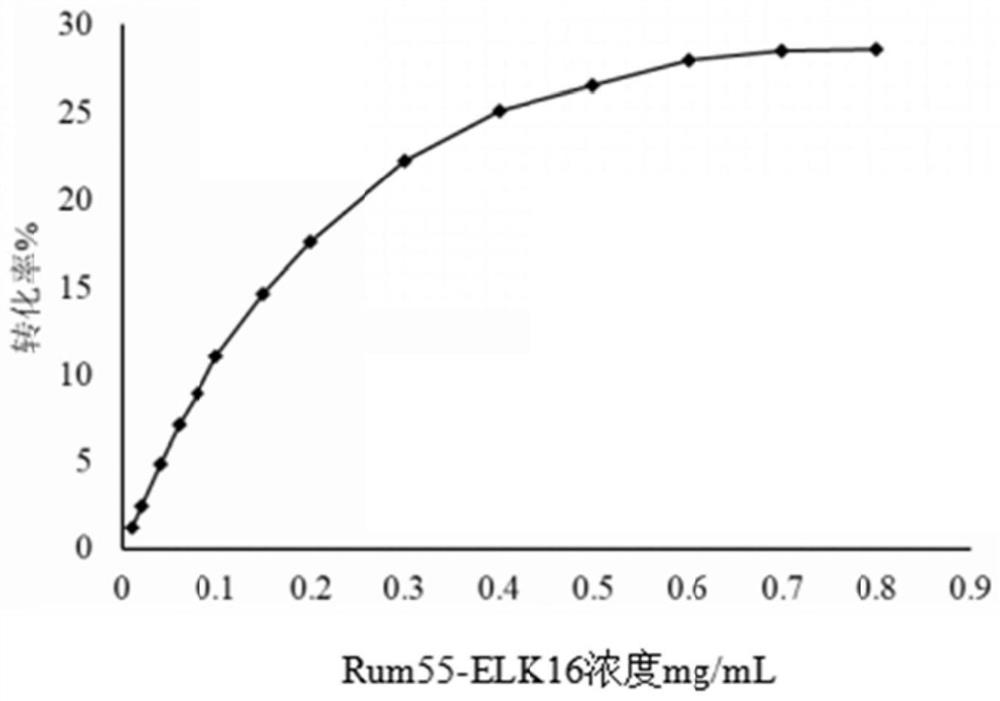
[0106] Example 2 Effects of different self-aggregating short peptides on the activity of D-psicose-3-epimerase active aggregates
[0107] According to the method of Example 1, the self-aggregating short peptide (ELK16, SEQ ID NO.2, 18A, SEQ ID NO.4, R18A, SEQ ID NO.5 and L6KD, SEQ ID NO.3)-PT-linker was constructed To the C-terminus of Rum55, D-psicose-3-epimerase active aggregates expressing different self-aggregating short peptide compositions were obtained: Rum55-L6KD aggregates (Rum55-PT-L6KD, SEQ ID NO.14 ), Rum55-PT-18A aggregate (Rum55-PT-18A, SEQ ID NO.15) and Rum55-R18A aggregate (Rum55-PT-R18A, SEQ ID NO.16) engineering bacteria: E.coli Rosetta (DE3) / pET30a-Rum55-L6KD, E.coli Rosetta(DE3) / pET30a-Rum55-18A, E.coli Rosetta(DE3) / pET30a-Rum55-R18A, as follows:
[0108] The gene sequences of L6KD, 18A and R18A are shown in SEQ ID NO.40, 41 and 42, respectively. Respectively with primers PT-L6KD-F (SEQ ID NO.44) and PT-L6KD-R (SEQ ID NO.45), PT-18A-F (SEQ ID NO.47) and ...
Embodiment 3
[0121] Example 3 Expression of Rum55-ELK16 in Bacillus subtilis
[0122] 1. Construction of plasmid pMA5-Rum55 ELK16
[0123] The pMA5 empty vector plasmid was double-digested with restriction endonucleases BamHI and MluI, and the digested vector fragment was gel-cut and recovered; using the Rum55-PT-ELK16 fragment obtained in Example 1 as a template, a Primer pMA5-BamHI-Rum55-F of pMA5 vector and restriction site sequence: agaatgcaaaaagtgaaatcaggg ggatcc ATGAACAAGATCGGAGTTCATTTTGGA (SEQ ID NO.29, the underlined base is the restriction endonuclease BamHI recognition site,) and pMA5-MluI-ELK-R: tcgaggtgaatttcgacctctaga acgcgt TTATTTCAGCTTTAATTCTAATTCCAGTTTTAA (SEQ ID NO.30, the underlined base is the restriction endonuclease MluI recognition site) was amplified to obtain the Rum55-PT-ELK16 target fragment (SEQ ID NO. 31), using the pEASY-UniSeamless Cloning and Assembly Kit (Beijing Quanshijin Biotechnology Co., Ltd.), the recovered vector fragment was combined with the amp...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com