Binuclear nitrogen-containing ligand IVB transition metal complex and application thereof in olefin high-temperature polymerization
A complex and IVB group technology, applied in the field of binuclear nitrogen-containing ligand IVB transition metal complexes and their high-temperature polymerization of olefins, can solve the problems of catalyst instability and catalyst structure destruction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
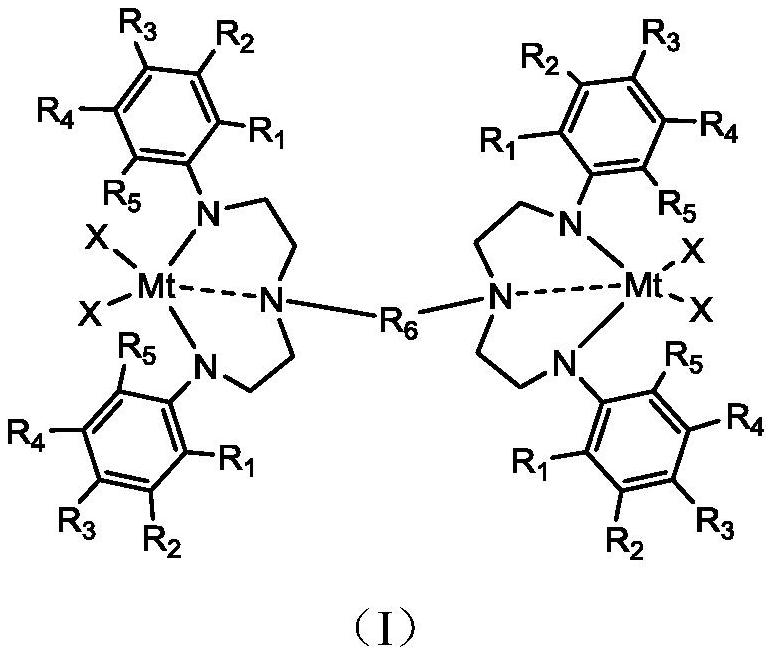
[0037] The second aspect of the present invention provides a method for preparing the complex provided by the first aspect of the present invention, comprising: combining an organic ligand with Mt(X) 4 Reaction, to provide the complex shown in formula (I).
[0038] Wherein, the chemical structure of organic ligand is as shown in formula (II):
[0039]
[0040] In formula (II), Mt is selected from IVB group metals, preferably, IVB group metals are selected from Ti, Zr or Hf; R 1 , R 2 , R 3 , R 4 , R 5 each independently selected from hydrogen, halogen, benzyl, linear, or branched C 1 ~C 6 the alkyl group; R 6C selected from straight chain or branched chain 1 ~C 10 Alkylene or C 3 ~C 10 of cycloalkylenes.
[0041] Mt(X) 4 In, X is selected from halogen, benzyl, linear or branched C 1 ~C 6 of alkyl.
[0042] In a specific embodiment, when X is selected from benzyl, the organic ligand shown in formula (II) can be combined with Mt(CH 2 Ph) 4 Reaction, to provi...
Embodiment 1
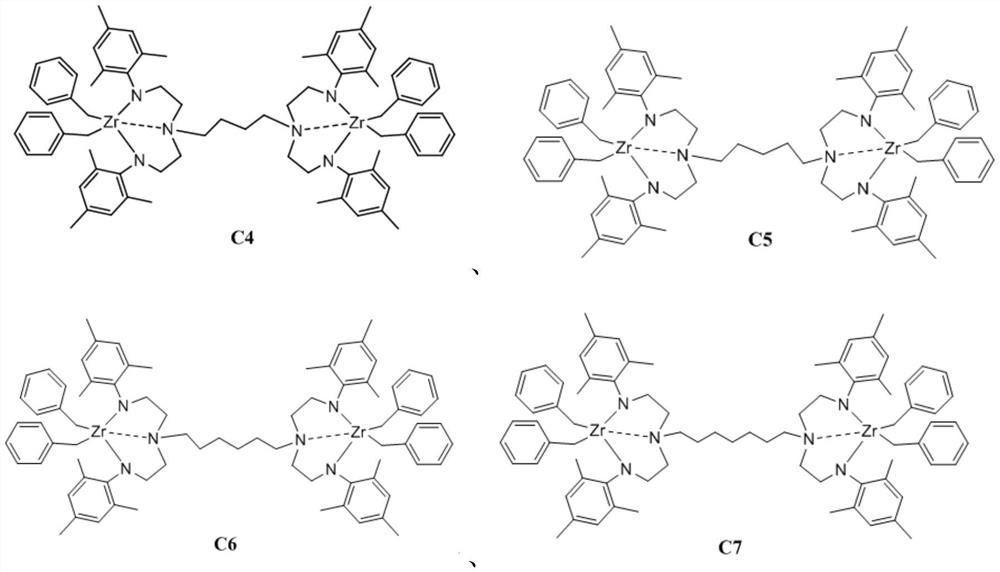
[0074] The synthetic steps of embodiment 1 C4
[0075] The synthetic route of C4 is as follows:
[0076]
[0077] 1) Ligand [(2,4,6-Me 3 C 6 h 2 ) NCH 2 CH 2 ] 2 N(CH 2 ) 4 N[CH 2 CH 2 N(2,4,6-Me 3 C 6 h 2 )] 2 (L4)synthesis
[0078] C4 bridged bisdiamine compound [HNCH 2 CH 2 ] 2 N(CH 2 ) 4 N[CH 2 CH 2 NH] 2 (a4) Synthetic method references (Inorg. Chem. 1992, 31, 3636-3646). Under nitrogen protection, a4 (3.13g, 12.0mmol), 2-bromo-mesitylene (10.90g, 48.0mol), tris(dibenzylideneacetone)dipalladium (0.11g, 0.12 mmol), rac-2,2′-bis(diphenylphosphine)-1,1′-binaphthyl (racemic BINAP) (0.22g, 0.36mmol), sodium tert-butoxide (6.92g, 42.0 mmol), and toluene (100 mL). While stirring, the temperature was raised to 100°C. Continue to react for 18h. The solvent was dried under vacuum, and the residue was dissolved in ether (90 mL), washed with water (3×25 mL), washed with saturated NaCl solution (18 g NaCl dissolved in 50 mL water), and dried by adding anh...
Embodiment 2
[0081] The synthetic steps of embodiment 2 C5
[0082] The synthetic route of C5 is as follows:
[0083]
[0084] 1) Ligand [(2,4,6-Me 3 C 6 h 2 ) NCH 2 CH 2 ] 2 N(CH 2 ) 5 N[CH 2 CH 2 N(2,4,6-Me 3 C 6 h 2 )] 2 (L5)synthesis
[0085] C5 bridged bisdiamine compound [HNCH 2 CH 2 ] 2 N(CH 2 ) 5 N[CH 2 CH 2 NH] 2 (a5) Synthetic method references (Inorg. Chem. 1992, 31, 3636-3646). Under nitrogen protection, a5 (3.29g, 12.0mmol), 2-bromo-mesitylene (10.90g, 48.0mol), tris(dibenzylideneacetone)dipalladium (0.11g, 0.12 mmol), rac-2,2′-bis(diphenylphosphine)-1,1′-binaphthyl (racemic BINAP) (0.22g, 0.36mmol), sodium tert-butoxide (6.92g, 42.0 mmol), and toluene (100 mL). While stirring, the temperature was raised to 100°C. Continue to react for 18h. The solvent was dried under vacuum, the residue was dissolved in ether (90 mL), washed with water (3×25 mL), washed with saturated NaCl solution (18 g NaCl dissolved in 50 mL water), and dried by adding anhydro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com