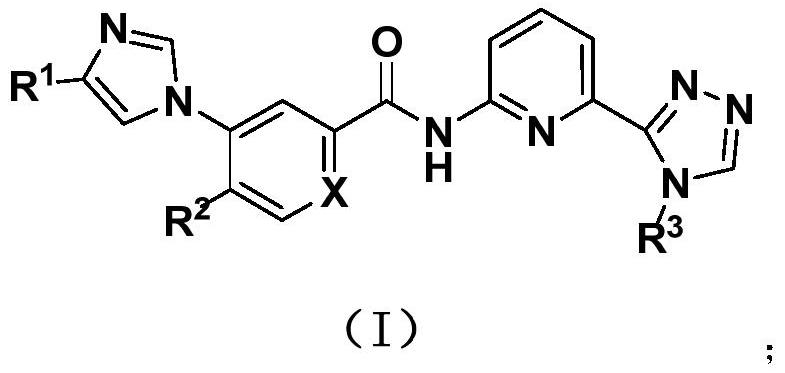
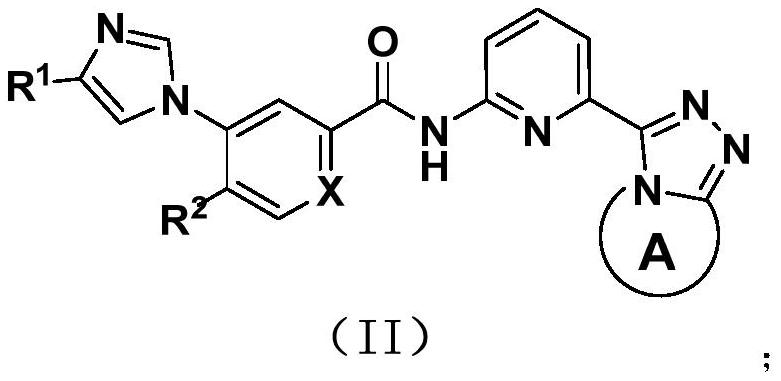
Aromatic heterocyclic amide compounds and uses thereof
A compound and hydrate technology, used in aromatic heterocyclic amide compounds as ASK1 inhibitors and their preparation, in the field of drug preparation, can solve the problems of poor compound drug-like properties, poor ASK1 target selectivity, and poor molecular-level activity. , to achieve the effect of improved activity, excellent liver metabolic stability, and good inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
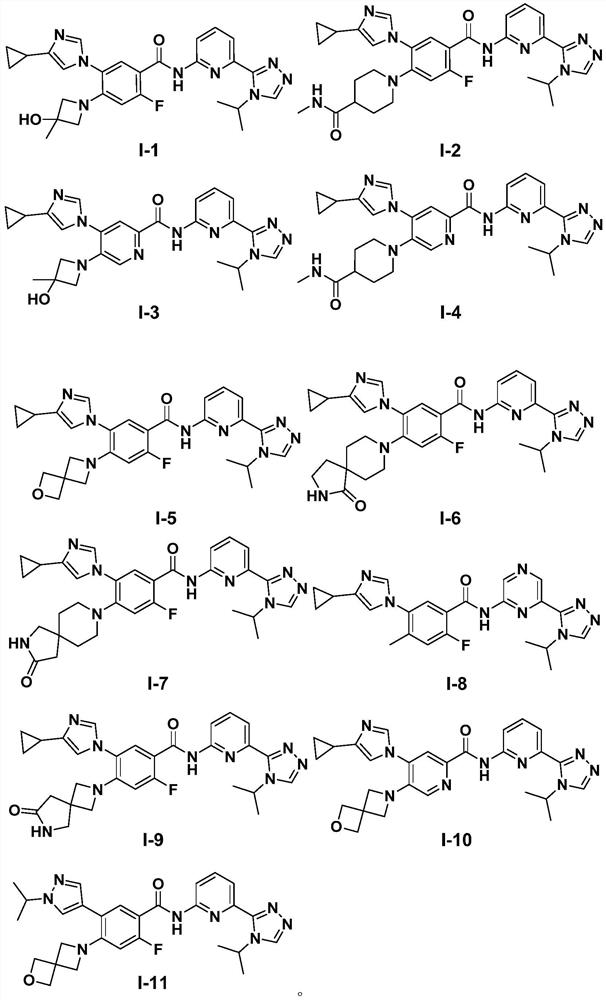
[0124] Embodiment 1: the synthesis of target compound I-1
[0125] 5-(4-cyclopropyl-1H-imidazol-1-yl)-2-fluoro-4-(3-hydroxyl-3-methylazetidin-1-yl)-N-(6-( 4-isopropyl-4H-1,2,4-triazol-3-yl)pyridin-2-yl)benzamide (compound I-1)
[0126]
[0127] The synthetic route of target compound I-1 is as follows:
[0128]
[0129] The first step: Synthesis of 2-fluoro-4-(3-hydroxy-3-methylazetidin-1-yl)-5-nitrobenzoic acid methyl ester (I-1B)
[0130] At 0°C, 3-methyl-3-hydroxyazetidine hydrochloride (0.63g, 5mmol) was added in batches to methyl 2,4-difluoro-5-nitrobenzoate (1.1g , 5mmol), DIEA (0.97g, 7.5mmol) in THF (15mL) solution. Stir at 0 °C for 1 h. After the reaction, add distilled water (50mL) to dilute, extract with ethyl acetate (50mL×3), combine the organic phases, wash the organic phase with saturated brine (70mL×2), separate the layers, and dry the organic phase with anhydrous sodium sulfate , filtered, concentrated, and the residue was purified by silica gel colu...
Embodiment 2
[0141] Embodiment 2: the preparation of target compound 1-2
[0142]
[0143] The synthetic route of target compound 1-2 is as follows:
[0144]
[0145] The first step: the synthesis of methyl 2-fluoro-4-(4-(methylcarbamoyl)piperidin-1-yl)-5-nitrobenzoate (I-2B)
[0146] At 0°C, N-methylpiperidine-4-carboxamide hydrochloride (5.18g, 29.0mmol) was added dropwise to methyl 2,4-difluoro-5-nitrobenzoate (6g, 27.6mmol ), DIEA (7.86g, 60.8mmol) in THF (80mL), stirred at 0°C for 2h. After the reaction, add water (80mL) to dilute, extract with ethyl acetate (80mL×3), combine the organic phases, wash the organic phase with saturated brine (70mL×2), separate the layers, and dry the organic phase with anhydrous sodium sulfate , filtered, concentrated, and the residue was purified by a silica gel column to obtain yellow solid 2-fluoro-4-(4-(methylcarbamoyl)piperidin-1-yl)-5-nitrobenzoic acid methyl ester (5g, yield rate 53.3%).
[0147] LC-MS, M / Z(ESI):340.3[M+H] + .
[0148]...
Embodiment 3
[0164] Embodiment 3: the preparation of target compound 1-3
[0165]
[0166] The synthesis route of the target compound is as follows:
[0167]
[0168] The first step: the synthesis of 2-chloro-4-(4-cyclopropyl-1H-imidazol-1-yl)-5-fluoropyridine (I-3B)
[0169] 8-Hydroxyquinoline (0.338g, 2.331mmol), cesium carbonate (11.39g, 35.0mmol), cuprous iodide (0.167g, 1.165mmol) were added to the solution containing 2-chloro-5-fluoro-4-iodine Pyridine (3g, 11.65mmol), 4-cyclopropyl-1H-imidazole (1.386g, 12.82mmol) in n-butyronitrile (50ml) solution. Under nitrogen protection, stir at 65°C for 16h. After the reaction, add distilled water (50mL) to dilute, extract with ethyl acetate (70mL×3), combine the organic phases, wash the organic phase with saturated brine (50mL), separate the layers, dry the organic phase with anhydrous sodium sulfate, and filter , concentrated, and the residue was purified by silica gel column (petroleum ether:ethyl acetate (V / V)=3:1) to give yellow ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com