Raloxifene hydrochloride tablet and preparation method thereof
A technology of raloxifene hydrochloride and tablets, which is applied in the field of raloxifene hydrochloride tablets and its preparation, can solve the problems of easy oxidation stability and photodegradation, and achieve good fluidity, good bulkiness, and application promising effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
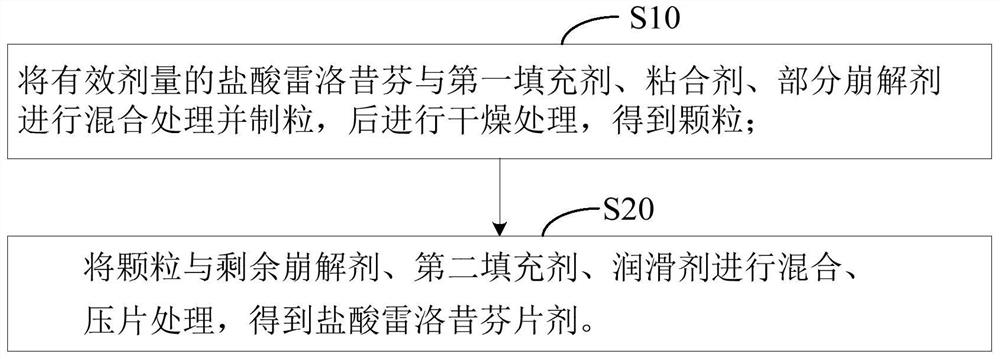
[0042] The second aspect of the embodiment of the present application provides a preparation method of raloxifene hydrochloride tablet, comprising the following steps:
[0043] S1: Mix and granulate an effective dose of raloxifene hydrochloride with the first filler, binder, and part of the disintegrant, and then dry to obtain granules;
[0044] S2: The granules, the remaining disintegrant, the second filler, and the lubricant are mixed and tabletted to obtain raloxifene hydrochloride tablets.
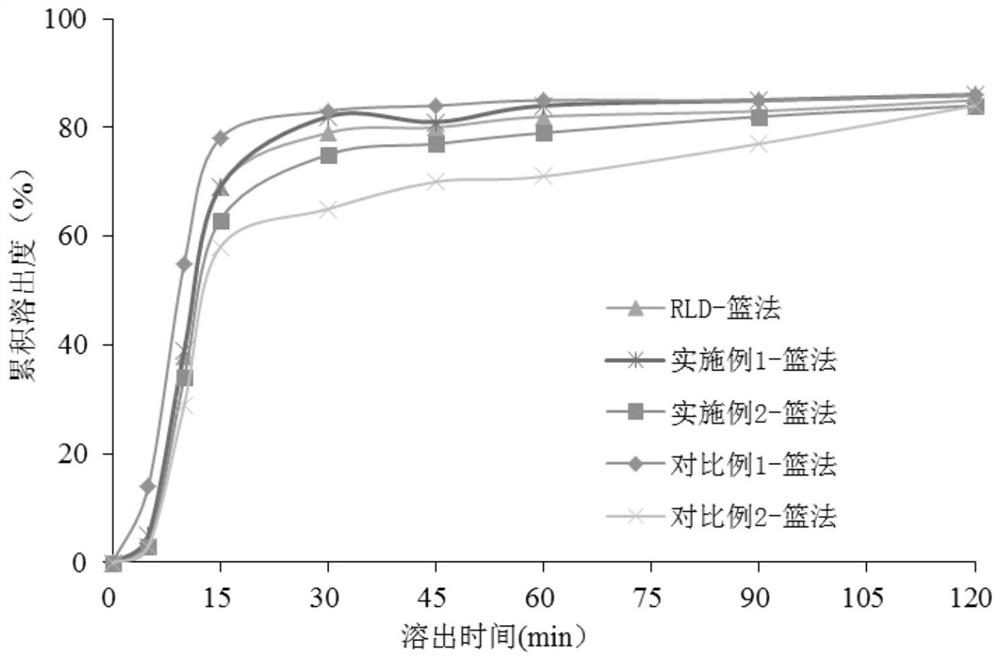
[0045] The preparation method of the raloxifene hydrochloride tablet provided in the embodiment of the present application uses anhydrous lactose as the first filler to carry out wet granulation, which can reduce the moisture of the granules, avoid the tablet being in a watery microenvironment, and improve the raloxifene hydrochloride. The stability of loxifene hydrochloride tablet; With titanium dioxide as coating layer raw material and by coating layer weight gain, can isolate tablet...
Embodiment 1
[0053] The present embodiment provides a kind of preparation method of raloxifene hydrochloride tablet, comprising:
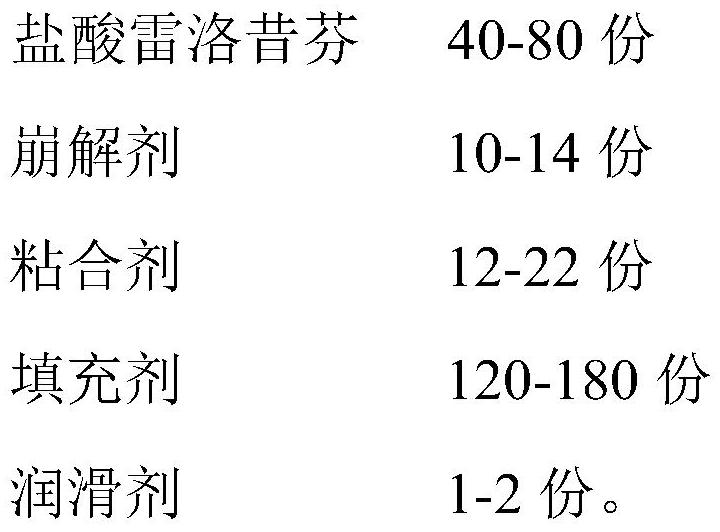
[0054] S10: Add 2.4g Tween 80 and 14.4g povidone into 43.2g water to make a suspension as a binder; add 60g non-solvated I crystalline raloxifene hydrochloride, 120g anhydrous lactose, 9g cross-linked polyvinyl chloride Vitone is added to the wet granulator for pre-mixing; then the binder is sprayed into the wet granulator through a peristaltic pump, atomized to make wet granules, and then sprayed and dried through a fluidized bed to control the dried granules Moisture content ≤ 1.5%, to obtain granulated dry granules; finally use a 0.8mm aperture sieve to sieve the granules to obtain granules;
[0055] S20: Add 30 g of spray-dried lactose monohydrate and 3 g of crospovidone to the granules prepared in step S10, and mix them in a mixer, then add 1.2 g of magnesium stearate for mixing, mix well and then press into tablets to obtain sulphate hydrochloride Loxife...
Embodiment 2
[0062] The present embodiment provides a kind of preparation method of raloxifene hydrochloride tablet, comprising:
[0063] S10: 2.4g Tween 80 and 18.0g povidone were added to 39.6g water to make a suspension as a binder; 60g non-solvated I crystalline raloxifene hydrochloride, 133.8g anhydrous lactose, 14g Add povidone into the wet granulator for pre-mixing; then directly add the binder into the wet granulator through the feeding port for granulation, and then pass through the drying oven for drying treatment, and control the moisture content of the dried granules to ≤1.5%, to obtain the Dry granules of granules; Finally, use a 0.8mm aperture sieve to sieve to obtain granules;
[0064] S20: Add 10 g of spray-dried lactose monohydrate to the granules prepared in step S10 and mix in a mixer, then add 1.8 g of magnesium stearate for mixing, mix evenly, and perform tablet compression to obtain raloxifene hydrochloride tablets;
[0065] S30: 14.4 g of the film coating premix is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com