Rupatadine fumarate tablets and preparation method thereof
A technology for rupatadine fumarate and tablets, which is applied in the field of pharmaceutical preparations, can solve problems such as poor stability, and achieve the effects of improving stability, being easy to implement, and reducing discoloration.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0020] The embodiment of the present invention provides a preparation method of rupatadine fumarate tablet, which comprises the following steps:
[0021] S1. Provide prescription amount of rupatadine fumarate, microcrystalline cellulose and other excipients;
[0022] S2. Mixing and granulating rupatadine fumarate with other excipients to obtain granules;
[0023] S3. Mixing and tableting the granules and microcrystalline cellulose to obtain rupatadine fumarate tablets.
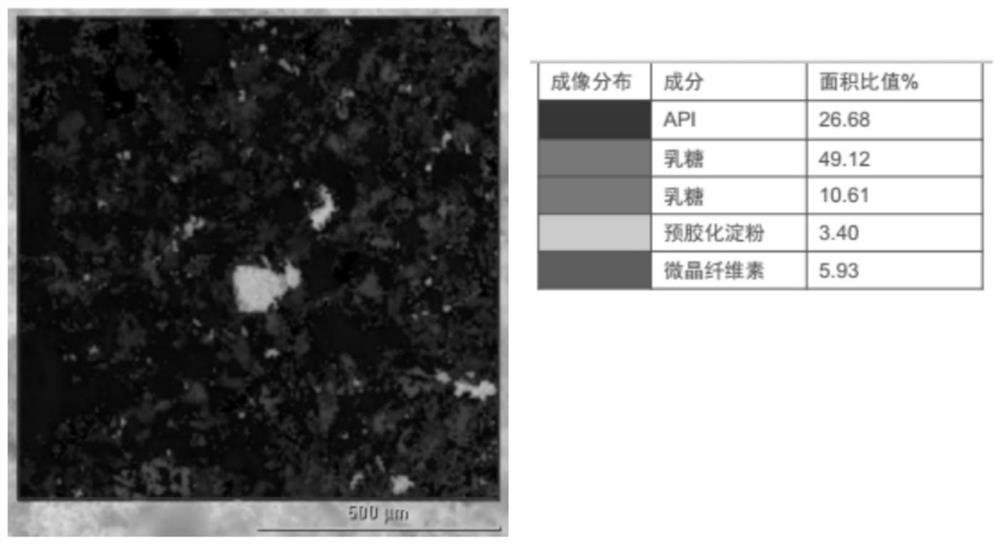
[0024] Microcrystalline cellulose is α-cellulose (obtained from plant fiber pulp) dilute inorganic acid solution through controlled hydrolysis, the hydrolyzed cellulose is filtered and purified, and the aqueous suspension is spray-dried to obtain dry, via particles. The impurities of microcrystalline cellulose are glucose, formaldehyde, nitrate and nitrite. The inventors of the present invention found in the research and development process that the hygroscopicity of the above-mentioned impurities will lead ...
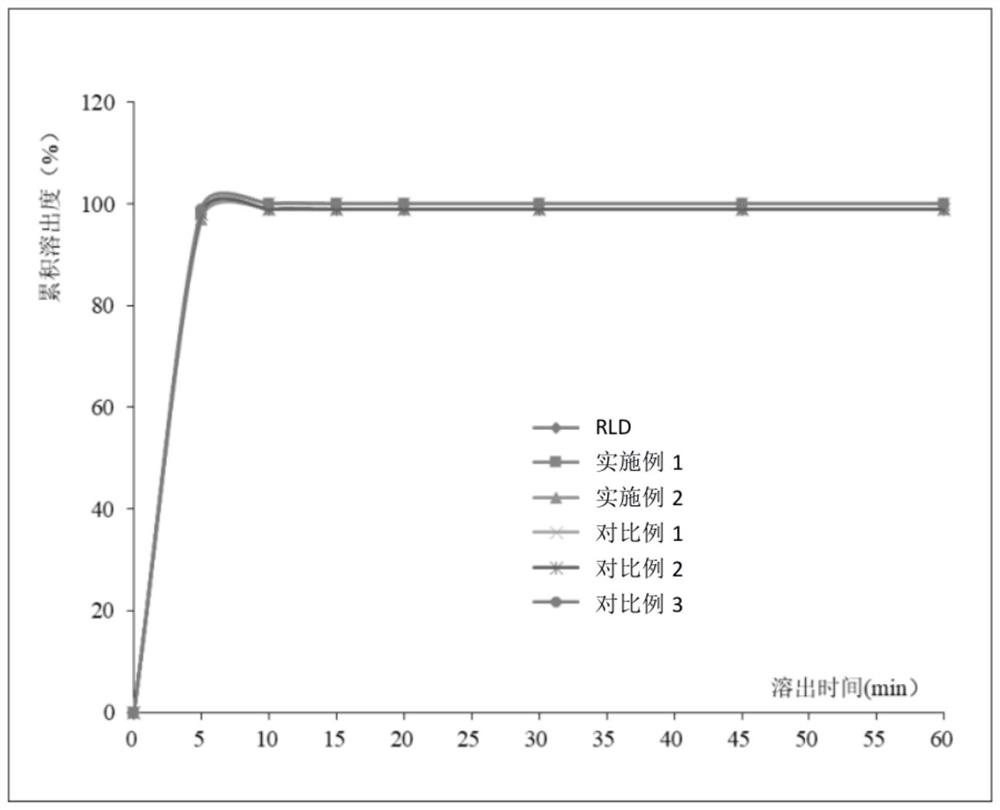
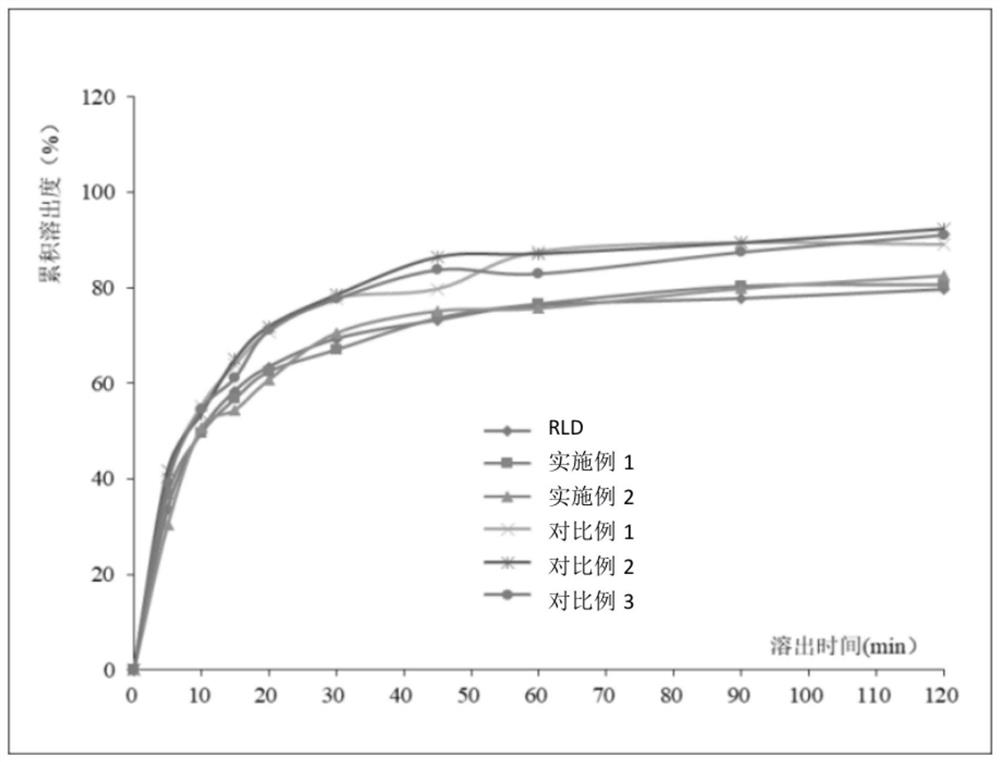
Embodiment 1
[0049] This embodiment provides a preparation method of rupatadine fumarate tablet, the steps are as follows:
[0050] (11) Rupatadine fumarate 12.8g (D 90 32 μm), lactose 60.1g, pregelatinized starch 10g, red iron oxide 0.025g and yellow iron oxide 0.075g were mixed uniformly in a wet granulator, and pregelatinized starch slurry 18g (solid content 10%) was added to make soft material, the drying temperature is 55°C, drying until the moisture content is ≤3.0%, and sizing with a 0.8mm aperture screen to obtain granules;
[0051] (12) Add 15 g of microcrystalline cellulose to the granules, and mix them uniformly in a mixer; then add 2 g of magnesium stearate, mix evenly, and perform tablet compression to obtain rupatadine fumarate tablets.
[0052] In the rupatadine fumarate tablets obtained in this example, the raw materials and contents used for every 1000 rupatadine fumarate tablets are shown in Table 1.
[0053] Table 1 Raw materials and content used in every 1000 rupatadi...
Embodiment 2
[0056] This embodiment provides a preparation method of rupatadine fumarate tablet, the steps are as follows:
[0057] (21) Rupatadine fumarate 12.8g (D 90 32 μm), 60.1 g of lactose, 10 g of pregelatinized starch, 0.025 g of red iron oxide and 0.075 g of yellow iron oxide were mixed uniformly in a wet granulator, and 18 g of corn starch slurry (solid content 10%) was added to make a soft material. The drying temperature is 55°C, drying until the moisture content is ≤3.0%, and sizing with a 0.8mm aperture sieve to obtain granules;
[0058] (22) Add 15 g of microcrystalline cellulose to the granules, and mix them uniformly in a mixer; then add 2 g of magnesium stearate, mix evenly, and perform tablet compression to obtain rupatadine fumarate tablets.
[0059] In the rupatadine fumarate tablets obtained in this example, the raw materials and contents used for every 1000 rupatadine fumarate tablets are shown in Table 2.
[0060] Table 2 Raw materials and content used in every 10...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com